Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Effects of hypertension and exercise in the brain and heart vasculome of rats
Time:2019-02-28
Number:9069
Jing Lan1,2, Shuzhen Guo2, Jingfei Shi1,2, Elga Esposito2, Emiri T. Mandeville2, Wenjun Deng2,3, Christiane D. Wrann2,4, MingMing Ning2,3, Xunming Ji1, Eng H. Lo2,3
Author Affiliations


- 1Department of Neurology and China-America Institute of Neuroscience, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA
- 3Clinical Proteomics Research Center, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, USA
- 4Cardiovascular Research Center, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, USA
Conditioning Medicine, 2018. 2(1):63-74.
Abstract
The concept of the vasculome posits that the microvascular network within each organ is unique and helps maintain organ homeostasis under normal conditions. Conversely, an unhealthy vasculome should contribute to organ pathophysiology during disease. In this study, we mapped the vasculome in brains and hearts from normotensive Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR), and asked whether exercise could renormalize the hypertensive vasculome. Microarrays were used to profile the transcriptome of microvessel endothelial cells isolated from brains and hearts of WKY and SHR. Sedentary rats were compared with those subjected to 8 weeks of treadmill running exercise. In sedentary rats, a significant number of genes were differentially expressed in SHR versus WKY, comprising many pathways previously implicated in the vasculome effects of hypertension. After treadmill running for 8 weeks, the brain and heart vasculomes in both WKY and SHR were altered, without any detectable changes in blood pressure. In SHR, exercise significantly renormalized subsets of genes and pathways that were initially affected by hypertension. These data suggest that some of the beneficial physiologic effects of exercise may involve the rescue of diseased vasculomes in brains and hearts. Further studies are warranted to explore vasculome databases for potential neurovascular and cardiovascular mechanisms, targets, and biomarkers in hypertension.
Abstract
The concept of the vasculome posits that the microvascular network within each organ is unique and helps maintain organ homeostasis under normal conditions. Conversely, an unhealthy vasculome should contribute to organ pathophysiology during disease. In this study, we mapped the vasculome in brains and hearts from normotensive Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR), and asked whether exercise could renormalize the hypertensive vasculome. Microarrays were used to profile the transcriptome of microvessel endothelial cells isolated from brains and hearts of WKY and SHR. Sedentary rats were compared with those subjected to 8 weeks of treadmill running exercise. In sedentary rats, a significant number of genes were differentially expressed in SHR versus WKY, comprising many pathways previously implicated in the vasculome effects of hypertension. After treadmill running for 8 weeks, the brain and heart vasculomes in both WKY and SHR were altered, without any detectable changes in blood pressure. In SHR, exercise significantly renormalized subsets of genes and pathways that were initially affected by hypertension. These data suggest that some of the beneficial physiologic effects of exercise may involve the rescue of diseased vasculomes in brains and hearts. Further studies are warranted to explore vasculome databases for potential neurovascular and cardiovascular mechanisms, targets, and biomarkers in hypertension.
Introduction
Endothelial cells play a central role in the physiology of all organ systems (Godo and Shimokawa, 2017) . Importantly, it is increasingly recognized that this vast vascular network or “vasculome” should also contribute to the pathophysiology of a wide range of diseases, including central nervous system (CNS) injuries and disorders (Bosetti et al., 2016; Gorelick et al., 2011; Hu et al., 2017; Koizumi et al., 2016). A recent study demonstrated that a significant number of genes and pathways are perturbed in brain and heart vasculomes that were mapped in mouse models of aging, hypertension, and diabetes (Guo et al., 2018). The importance of this concept is underscored by the observations that (i) the vasculome is perturbed after stroke and brain trauma (Bosetti et al., 2016; Guo et al., 2016; Zhang et al., 2016); (ii) vascular co-morbidities significantly contribute to development of almost all CNS disorders (Brown and Thore, 2011; Gorelick et al., 2011; Guo et al., 2018); and (iii) reduction of vascular risk factors and/or vascular therapeutics appear to be broadly effective in stroke, trauma, and neurodegeneration (Hasnain and Vieweg, 2014; Pistoia et al., 2016; Trigiani and Hamel, 2017).
In contrast to the difficulties in finding clinically effective “neuroprotective drugs” for CNS disease, some of the stronger signals may be observed in the vasculome. Diet and lifestyle strategies that improve vascular health have been correlated with reductions in risk of stroke and dementia (Boehme et al., 2017; Cassidy et al., 2016; Teuschl et al., 2017). Exercise is thought to improve cardiovascular, cerebrovascular, and neuronal health (Che and Li, 2017; Nishijima et al., 2016). Recent reviews and perspectives hypothesized that the beneficial effects of exercise may be mediated in part by its effects on the brain endothelium (Trigiani and Hamel, 2017; Zimmer and Bloch, 2015). If so, then one should be able to systematically document the effects of disease in the vasculome and show that therapies can rescue these disruptions in patterned and meaningful ways.
In this proof-of-principle study, we compared normotensive Wistar-Kyoto rats (WKY) versus spontaneously hypertensive rats (SHR), and asked whether and how exercise might renormalize the hypertensive brain and heart vasculome in these rat models.
Materials and Methods
Animal models: All studies followed animal protocols approved by a Subcommittee for Research Animal Care of the Massachusetts General Hospital IACUC (Institution Animal Care and Use Committee), and were consistent with the NIH Guide for the Care and Use of Laboratory Animals. Nine week old male Wistar-Kyoto rats (WKY) and male Spontaneously Hypertensive Rats (SHR) were used as normotensive and hypertensive animal models, respectively (Charles River Company). By this age of nine weeks, male SHR have significantly elevated blood pressure (160/100 mmHg) compared to WKY rats (120/80 mmHg). Previous studies also confirmed the presence of endothelial dysfunction in SHR at this age (Anishchenko et al., 2015). Rats were randomly divided into four groups: exercised SHR, sedentary SHR, exercised WKY, and sedentary WKY (n = 6 rats per group; 3 for microarrays and 3 for physiological monitoring, behavioral testing, and real time RT-PCR confirmation).
Exercise protocols and physiologic testing: It has been reported that plasma corticosterone (an indirect marker of overall stress response) is not altered by mild exercise (Inoue et al., 2015). A similar mild exercise protocol was used in our present study. Rats were habituated to treadmill running (Columbus Instruments, Columbus, OH) with no inclination, followed by eight weeks of exercise. Running was performed five days a week (Monday, Tuesday, Thursday, Friday and Saturday) between 18:00-22:00 in order to match the nocturnal periods of rodents. In the habituation phase, rats ran on the treadmill with gradually increased speed and duration, from 5 m/min for 15 min to 10 m/min for 15 min on the first day, up to 10 m/min for 10 min to 15 m/min for 50 min on the last day. In the exercise phase, rats ran at 10 m/min for 5 min plus 15 m/min for 55 min, for a total of 60 min during each exercise session. Sedentary rats were put on a non-moving treadmill for one hour, five days a week. Rats were sacrificed three days after the exercise protocol, in part to minimize potential acute effects of exercise stress (Kant et al., 1987). Weight was measured before exercise every Monday for nine weeks in total. Blood pressure measurement and Y-maze test were carried out before, during (the 4th week) and after the exercise plan, respectively. Blood pressure was measured with a noninvasive method via tail cuffs (CODA, Kent Scientific, Torrington, CT). Behavioral testing was performed using the standard Y maze; total number of entries and the number of entries into the correct arm were recorded, and the ratio of entries into the correct arm was calculated (Kouemou et al., 2017).
Purification of endothelial cells: Following our previously published methods (Guo et al., 2012), endothelial cells were isolated from rat brain cortex and heart. Briefly, rat brains and hearts were extracted after cardiac perfusion with 40 ml phosphate buffered saline (Invitrogen, Carlsbad, CA). Cortical tissues were minced after removing the olfactory bulb, brain stem, subcortical tissue, and meninges. Cardiac tissues were minced after removing large blood vessels. Samples were digested in 2 mg/ml Collagenase A (Worthington, Lakewood, NJ) at 37°C for 30-40 min with vigorous shaking, then mechanically dissociated by titrating through 14 Gauge needles, and filtered through a 70 μM cell strainer (Becton Dickinson Labware, Bedford, MA). Digested tissue was pelleted and washed with Hank’s balanced salt solution (HBSS) by centrifugation at 400 xg for 8 min at 4°C, and resuspended in a small volume of HBSS to incubate with anti-Platelet and endothelial cell adhesion molecule 1(PECAM1) antibody-coated Dynabeads. Four microgram of Biotin mouse anti-rat PECAM1 antibody (BD Biosciences, San Jose, CA) was conjugated with 40 μl Dynabeads M-280 Streptavidin (Invitrogen, Carlsbad, CA) as recommended by the manufacturer, before incubation with each tissue homogenate. After 40 min at 4°C with gentle rotation, the antibody-bead-bound endothelial cells were recovered and washed in HBSS with a magnetic separator, then for RNA preparation with RNeasy Micro Plus kit (Qiagen)
Transcriptome profiling with microarray and data analysis: Rat Gene 2.0 ST array was used to map the transcriptome in rat cerebral and cardiac vasculomes. All RNA samples had RNA Integrity number (RIN) scores larger than 7.0, measured by RNA Piko kit on Bioanalyzer 2001 (Agilent). Arrays were normalized together using the Robust Multiarray Average (RMA) algorithm (Gautier et al., 2004; Irizarry et al., 2003) included in the Bioconductor software suite (version 2.12) (Gentleman et al., 2004), and a CDF (Chip Definition File) for Entrez Gene ID-specific probeset mapping (17.0.0) (http://brainarray.mbni.med.umich.edu/Brainarray/Database/CustomCDF) (Dai et al., 2005). All arrays passed the technical quality assessment with Relative Log Expression (RLE) and Normalized Unscaled Standard Error (NUSE), indicating that all samples were of similar quality, suitable for further analysis. Differential expression was assessed using the moderated (empirical Bayesian) t-test implemented in the limma package (version 3.14.4). Correction for multiple hypothesis testing was accomplished using the Benjamini-Hochberg false discovery rate (FDR). Mouse homologs of rat genes were identified using HomoloGene (version 68) (Coordinators, 2013). Expression values above array-wise median (~5 in log2 units) and standard of p < 0.05, plus fold change > 1.2 or < -1.2 were used to filter out the differentially expressed genes (DEGs). Biological functions of DEGs were analyzed on David Bioinformatics Resources (https://david.ncifcrf.gov) (Huang da et al., 2009). Gene Set Enrichment Analysis (GSEA, version 2.2.1) (Subramanian et al., 2007; Subramanian et al., 2005) was used to identify biological pathways that are coordinately up- or down-regulated within each pairwise comparison, based on Reactome database (Fabregat et al., 2018). Standard of p < 0.05 was used to choose the Reactome pathways.
Real-Time quantitative reverse transcription PCR (qRT-PCR): Expression of cell type markers in our endothelial preparation and corresponding organs were measured by qRT-PCR. The relative expressions of selected DEGs were also confirmed by qRT-PCR in a new set of RNA samples. First strand cDNA was synthesized with QuantiTect reverse transcription system (Qiagen). PCR reactions were carried out with Taqman primers and Fast universal master mix, using 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The fold change of each gene was measured with comparative Ct method (2-ΔΔCt), normalized against the level of housekeeping gene hprt1 (Hypoxanthine phosphoribosyltransferase 1). Endothelial cell markers used were Pecam1, cadherin 5 (CDH5), and TEK receptor tyrosine kinase (TIE-2). Microtubule associated protein 2 (MAP-2) and neurogranin (NRGN) were used as neuronal markers. Aquaporin-4 (AQP4) and glial fibrillary acidic protein (GFAP) was used as astrocyte markers. Myelin basic protein (Mbp) was used as an oligodendrocyte marker. Allograft Inflammatory Factor 1 (AIF-1) was used as a microglia marker. Myosin heavy chain 6 (Myh6) and NK2 homeobox 5 (Nkx2-5) were used as cardiac myocyte markers.
Statistical analysis: Data are expressed as mean ± SD, analyzed by two-way repeated measurement ANOVA followed by Bonferroni t-test. Values of p less than 0.05 were considered statistically significant.
Results
Quality control
All RNA samples had RNA Integrity number (RIN) scores greater than 7.0. PCR results showed that samples from brain (Figure 1A) and heart (Figure 1B) were enriched in endothelial gene expression compared with other cell types. Based on our previous vasculome studies, additional representative genes were selected (Bcl2l11, Ccl2, Fmo2, Irf9, Mx1, Parp14, Perp, Per2, Rsad2, Serf1, Snca, Usp18, and Zbtb16) and results from microarrays were compared with PCR. There was good correlation between PCR and microarray data (R2 = 0.71) (Figure 1C).
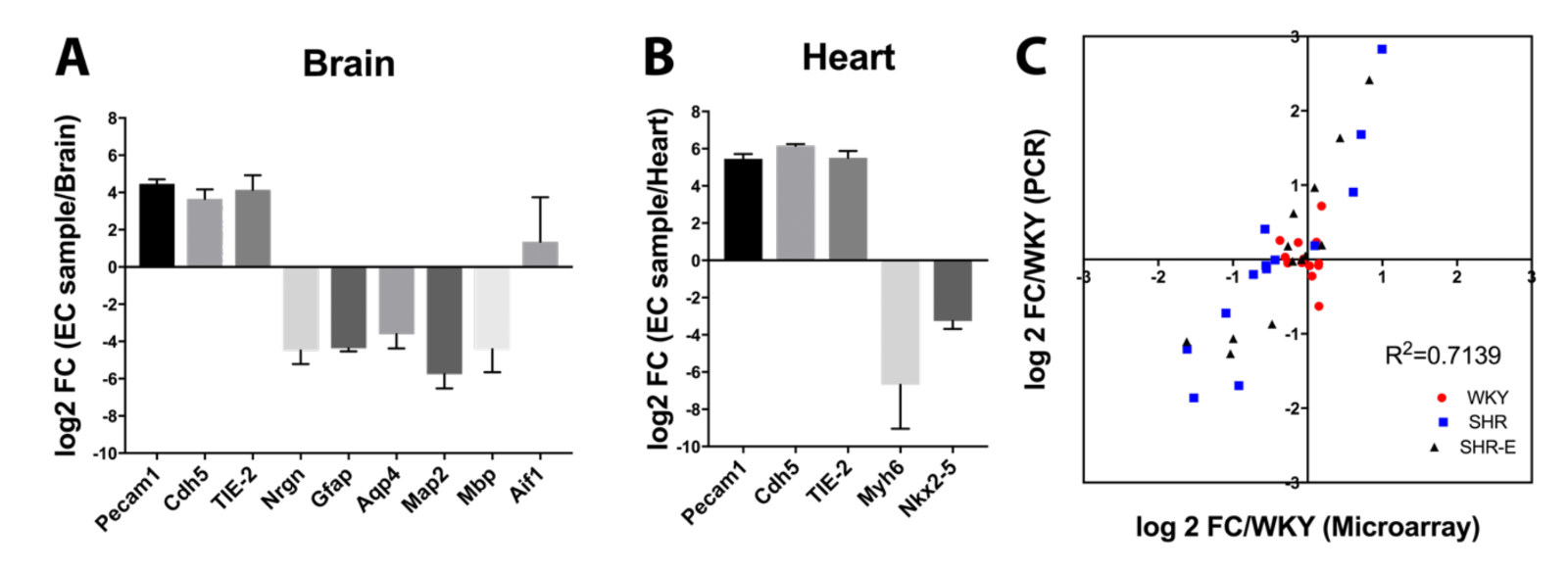
In a new window | Download PPT
Figure 1: Comparison of different cell type markers between endothelial cell samples and their original organ tissue in (A) brain or (B) heart. (C) Comparison of some differentially expressed genes between RT-PCR and microarray results.
Effects of hypertension on rat brain and heart vasculomes
Principal component analysis suggests differences between brain versus heart are larger than differences between WKY versus SHR, indicating that organ effects on the vasculome are greater than the effects of hypertension or exercise (Figure 2A). Nevertheless, both brain and heart vasculomes of SHR were significantly different from WKY, with many DEGs (Figure 2B-C).
In spite of overall organ-specific responses, 323 DEGs were shared in the brain and heart of SHR, and the majority of them (273) had the same direction of change in both organs (Figure 2D). GSEA analysis based on the Reactome database showed that there were many altered pathways in the SHR vasculome (Figure 2E-F). In the SHR brain, signal transduction and immune responses were downregulated, whereas metabolism pathways showed a mixed response with both upregulation and downregulation. In the SHR heart, cell cycle pathways were downregulated.
Compared to WKY, 17 common Reactome pathways were changed in both brain and heart vasculomes of SHR, and many of them showed the same direction of change in both organs (Figure 2G), including interferon signaling, interleukin signaling, and cytokine signaling (Table 1). These similarities in both organs suggest common effects of hypertension on the endothelium.
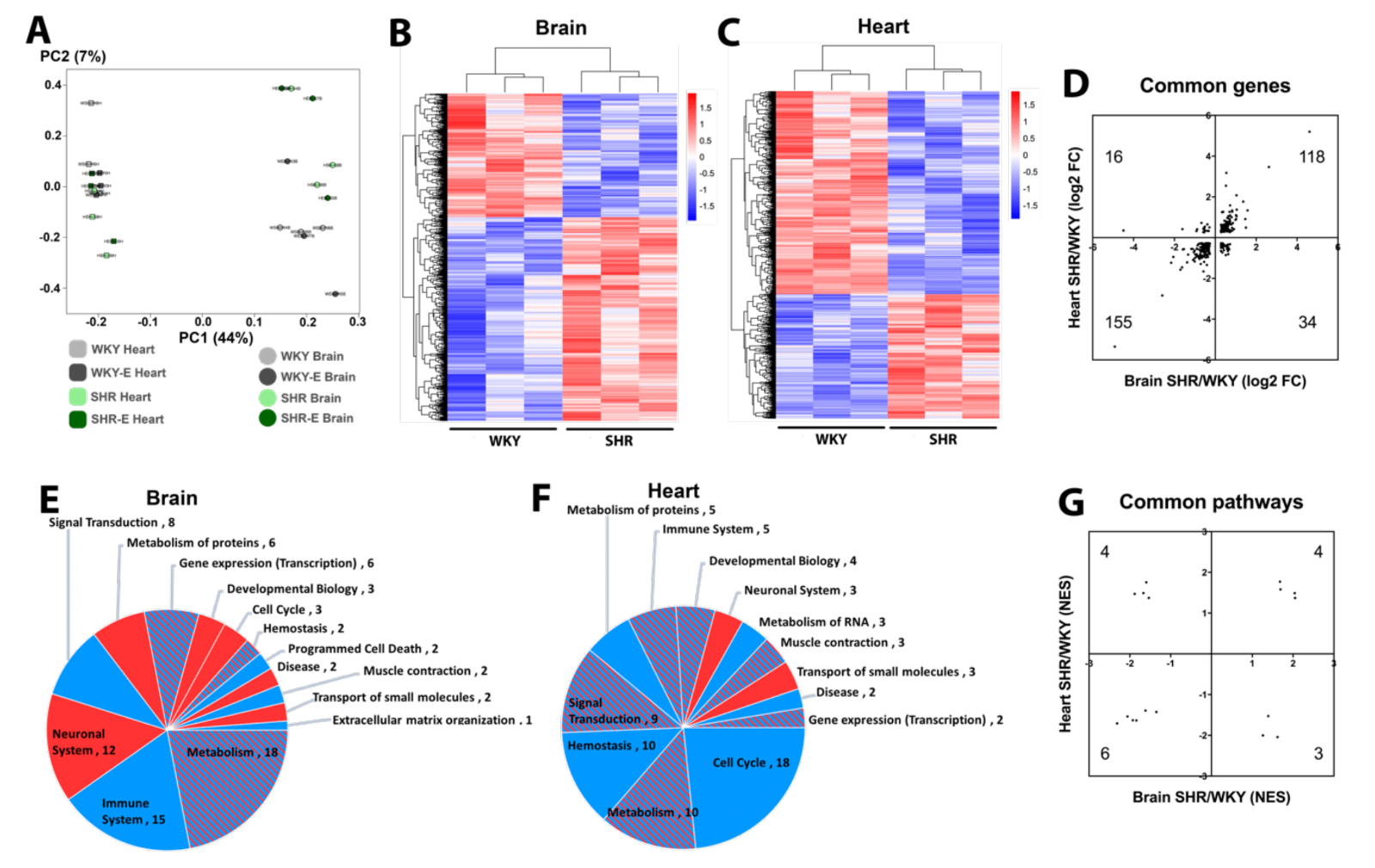
In a new window | Download PPT
Figure 2: (A) Principal component analysis of vasculomes from 4 groups. Heatmap of hypertension-induced DEGs in SHR (B) brain and (C) heart. (D) Distribution of fold-change directions for common DEGs in brain and heart of SHR. Pie chart of Reactome pathways induced by hypertension in (E) SHR brain and (F) SHR heart; red for upregulation, blue for downregulation, and shaded for mixed directions of change. (G) Distribution of change directions for common Reactome pathways in brain and heart of SHR.
Table 1: Common Reactome pathways changed by hypertension in brain and heart of SHR vasculomes.
|
Brain |
Heart |
|||||
|
Reactome, SHR/WKY |
size |
NES |
p |
NES |
p |
|
|
Neuronal system |
260 |
2.05 |
0.000 |
1.37 |
0.016 |
|
|
Potassium channels |
93 |
2.04 |
0.000 |
1.49 |
0.014 |
|
|
Interaction between L1 and ankyrins |
21 |
1.69 |
0.014 |
1.58 |
0.027 |
|
|
CREB phosphorylation through the activation of Ras |
23 |
1.68 |
0.023 |
1.77 |
0.014 |
|
|
G alpha (S) signaling events |
112 |
-1.35 |
0.041 |
-1.43 |
0.018 |
|
|
Signaling by ILs |
100 |
-1.62 |
0.005 |
-1.39 |
0.036 |
|
|
Smooth muscle contraction |
15 |
-1.84 |
0.009 |
-1.63 |
0.032 |
|
|
Cytokine signaling in immune system |
218 |
-1.93 |
0.000 |
-1.63 |
0.000 |
|
|
Interferon signaling |
117 |
-2.06 |
0.000 |
-1.54 |
0.004 |
|
|
Interferon gamma signaling |
48 |
-2.31 |
0.000 |
-1.71 |
0.004 |
|
|
Cholesterol biosynthesis |
21 |
1.62 |
0.029 |
-2.04 |
0.000 |
|
|
Mitotic G2 - G2/M phases |
68 |
1.39 |
0.045 |
-1.53 |
0.023 |
|
|
Cell cycle mitotic |
275 |
1.26 |
0.041 |
-2.00 |
0.000 |
|
|
GPCR lignad binding |
352 |
-1.53 |
0.000 |
1.37 |
0.004 |
|
|
Complement cascade |
23 |
-1.60 |
0.044 |
1.76 |
0.008 |
|
|
Class A/1 Rhodopsin-like receptors |
259 |
-1.67 |
0.000 |
1.49 |
0.006 |
|
|
Peptide ligand binding receptors |
156 |
-1.88 |
0.000 |
1.47 |
0.006 |
|
Comparison of hypertensive vasculomes in rats versus mice
In the present study, we used SHR as a model for hypertension, but there are no perfect models for human disease. Therefore, we compared our present SHR vs WKY findings with a previously published dataset of hypertensive BPH vs normotensive BPN mice, using the same selection criteria (Guo et al., 2018). As expected, there was overlap between the mouse and rat vasculome response to hypertension (Table 2). In the brain, there were 79 common DEGs and 13 common Reactome pathways. In the heart, there were 108 common DEGs and 9 common Reactome pathways. Nevertheless, species differences may still be important since some of these genes and pathways appeared to respond in opposite directions (Figure 3A-D). Some genes and pathways were present in both brain and heart, and present in both rats and mice (Table 2). This core subset of the hypertensive vasculome should represent highly conserved responses of endothelium to disease, and may warrant further dissection.
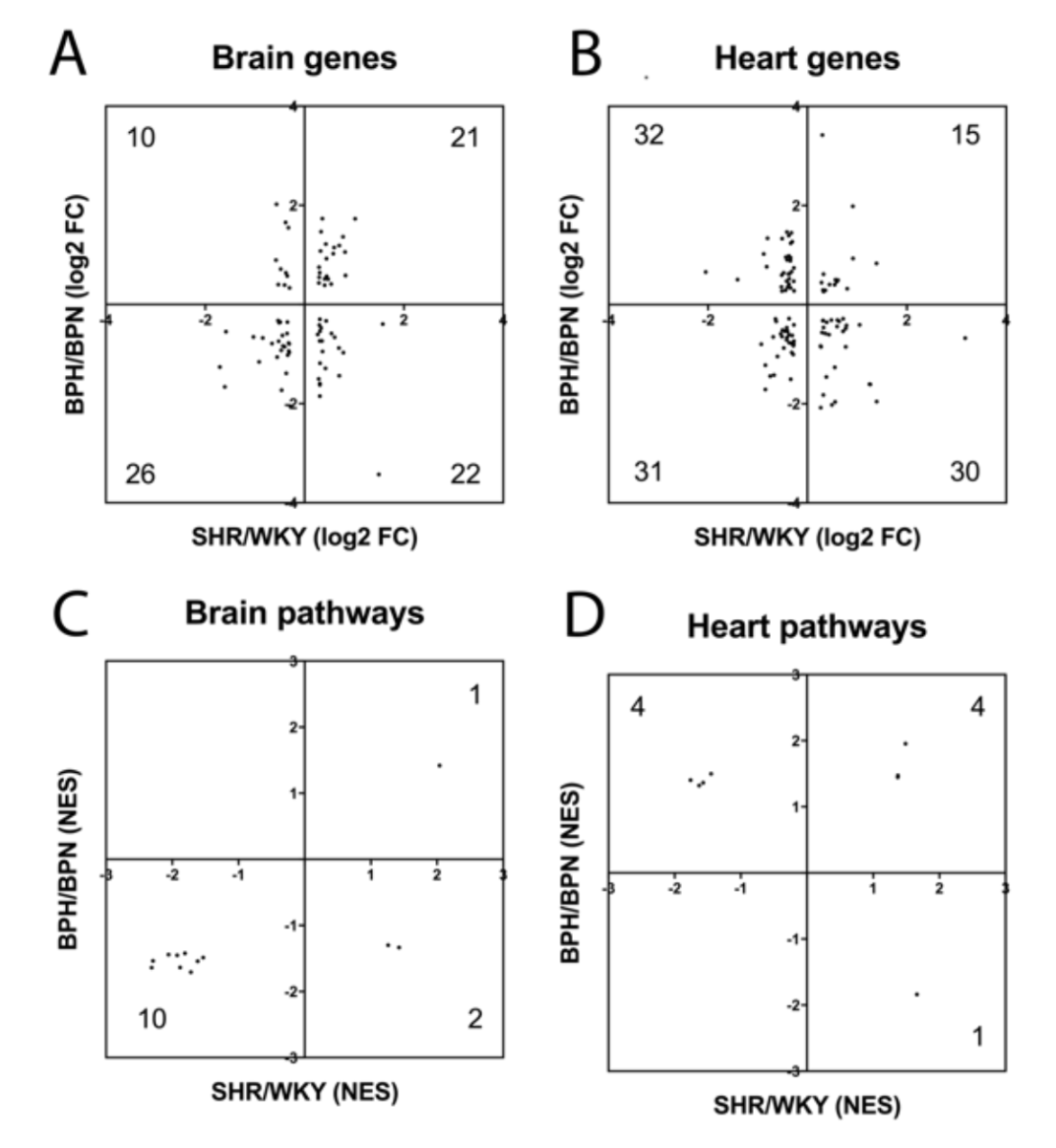
In a new window | Download PPT
Figure 3: Distribution of fold-change directions for common hypertension DEGs in SHR-vs-WKY and BPH-vs-BPN in (A) brain and (B) heart. Distribution of change directions for hypertension-induced common Reactome pathways in SHR-vs-WKY and BPH-vs-BPN in (C) brain and (D) heart.
Table 2: Comparisons between hypertensive rat SHR and hypertensive mouse BPH.
(A) Numbers of significantly changed DEGs and pathways:
|
DEG |
Reactome |
||||||
|
Hypertension |
SHR |
BPH |
Common in species |
|
SHR |
BPH |
Common in species |
|
Brain |
1318 |
1206 |
79 |
82 |
25 |
13 |
|
|
Heart |
1422 |
978 |
108 |
77 |
44 |
9 |
|
|
Common in organs |
323 |
231 |
5 |
17 |
9 |
4 |
|
(B) List of commonly changed DEGs:
|
Rat |
SHR, Brain |
SHR, Heart |
Mouse |
BPH, Brain |
BPH, Heart |
|||||
|
Symbol |
Gene ID |
FC |
p |
FC |
p |
Gene ID |
FC |
p |
FC |
p |
|
Dapk1 |
306722 |
1.29 |
0.041 |
-1.21 |
0.013 |
69635 |
-1.93 |
0.032 |
-1.74 |
0.002 |
|
Lig4 |
290907 |
2.82 |
0.000 |
1.41 |
0.033 |
319583 |
-10.76 |
0.001 |
-4.06 |
0.005 |
|
Setd7 |
689954 |
1.25 |
0.023 |
1.22 |
0.008 |
73251 |
2.11 |
0.002 |
1.36 |
0.004 |
|
Trim16 |
303214 |
2.02 |
0.000 |
1.88 |
0.000 |
94092 |
3.31 |
0.002 |
3.96 |
0.000 |
|
Uggt2 |
361091 |
1.62 |
0.005 |
2.40 |
0.000 |
64435 |
-1.44 |
0.007 |
-1.61 |
0.002 |
(C) List of commonly changed Reactome pathways:
|
Rat |
SHR, Brain |
SHR, Heart |
Mouse |
BPH, Brain |
BPH, Heart |
|||||
|
Reactome |
size |
NES |
p |
NES |
p |
size |
NES |
p |
NES |
p |
|
Potassium channels |
93 |
2.04 |
0.000 |
1.49 |
0.014 |
58 |
1.42 |
0.031 |
1.95 |
0.001 |
|
GPCR ligand binding |
352 |
-1.53 |
0.000 |
1.37 |
0.004 |
144 |
-1.49 |
0.002 |
1.47 |
0.002 |
|
Class A/1 Rhodopsin-like receptors |
259 |
-1.67 |
0.000 |
1.49 |
0.006 |
90 |
-1.55 |
0.005 |
1.47 |
0.013 |
|
Cytokine signaling in immune system |
218 |
-1.93 |
0.000 |
-1.63 |
0.000 |
178 |
-1.45 |
0.004 |
1.32 |
0.032 |
Physiologic effects of exercise in SHR and WKY
During the 10 weeks of the experiment, all rats continued to grow with ad libitum access to food and water in standard housing conditions. Exercise did not affect weight gain in WKY, but slightly decreased body weight in SHR toward the end of the study period (Figure 4A-B). Exercise did not significantly affect blood pressure. WKY remained within the normal range of approximately 110-120 mmHg. SHR remained hypertensive, approximately 160 mmHg (Figure 4C-D).
To indirectly assess cognitive function, all rats were subjected to Y-maze testing before, during (at 4 wks), and after the end (at 8 wks) of the exercise sessions. Quantitation of total entries suggested that SHR may be more spontaneously hyperactive compared to WKY (Figure 4E-F). Exercise did not change entry numbers in WKY. However, at the end of the 8 wk exercise protocol, total entry numbers in SHR were significantly reduced (Figure 4F). No significant effects were detected in alternation percentages, an indicator of spatial memory (Figure 4G-H).
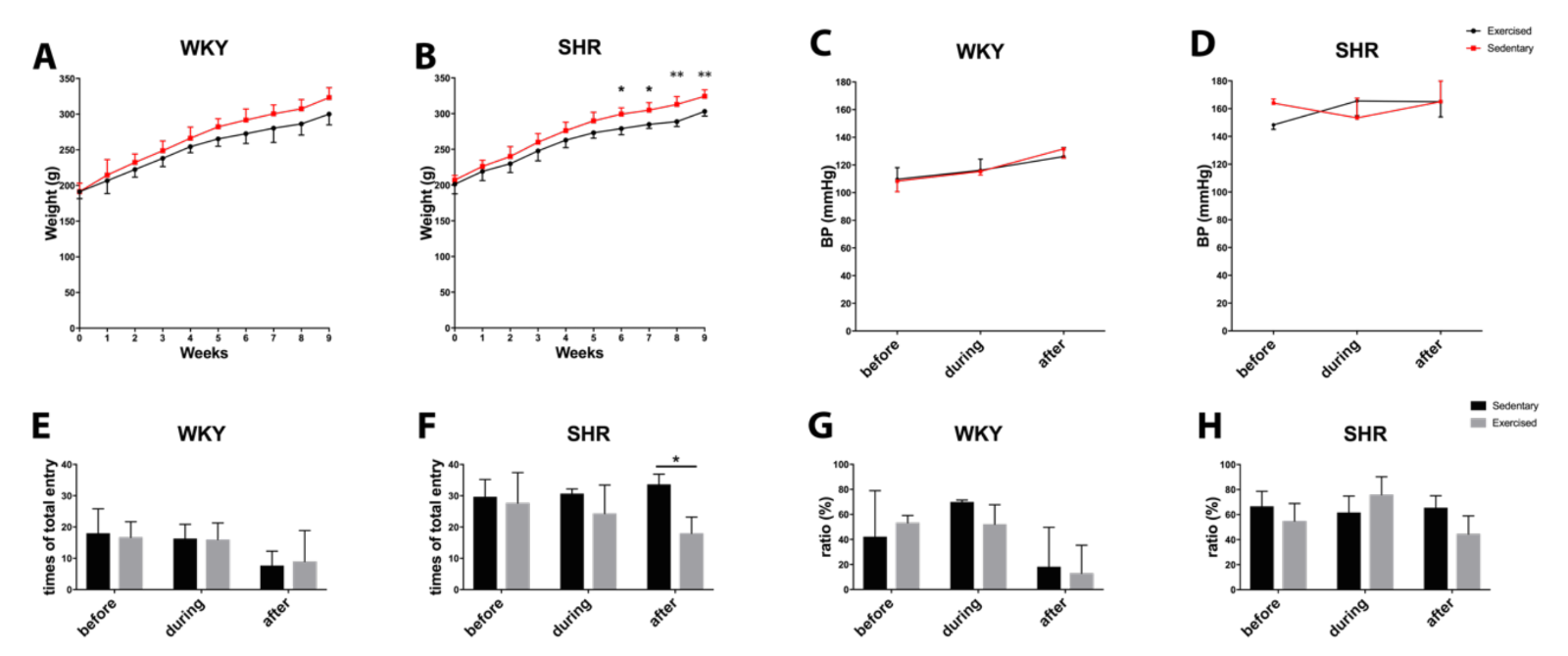
In a new window | Download PPT
Figure 4: Body weight during the process of exercise in (A) normotensive WKY and (B) hypertensive SHR. Blood pressure during the process of exercise in (C) normotensive WKY and (D) hypertensive SHR. Total entry numbers in Y-maze test for (E) WKY and (F) SHR. Ratio of correct entry (percentage) in Y-maze for (G) WKY and (H) SHR. * for p < 0.05 and ** for p < 0.01.
Effects of exercise on the brain and heart vasculomes
Exercise clearly altered brain and heart vasculomes in both normotensive WKY and hypertensive SHR (Figure 5A-D). In the brain, there were 2 common DEGs and 15 common Reactome pathways in WKY and SHR (Table 3). In the heart, there were 9 common DEGs and 13 common Reactome pathways in WKY and SHR (Table 3). Of potential interest was the fact that exercise modified the hypertensive vasculome in a consistent way. There were 27 common DEGs in brains and hearts of exercised SHR. All of these were upregulated by exercise (Figure 5E).
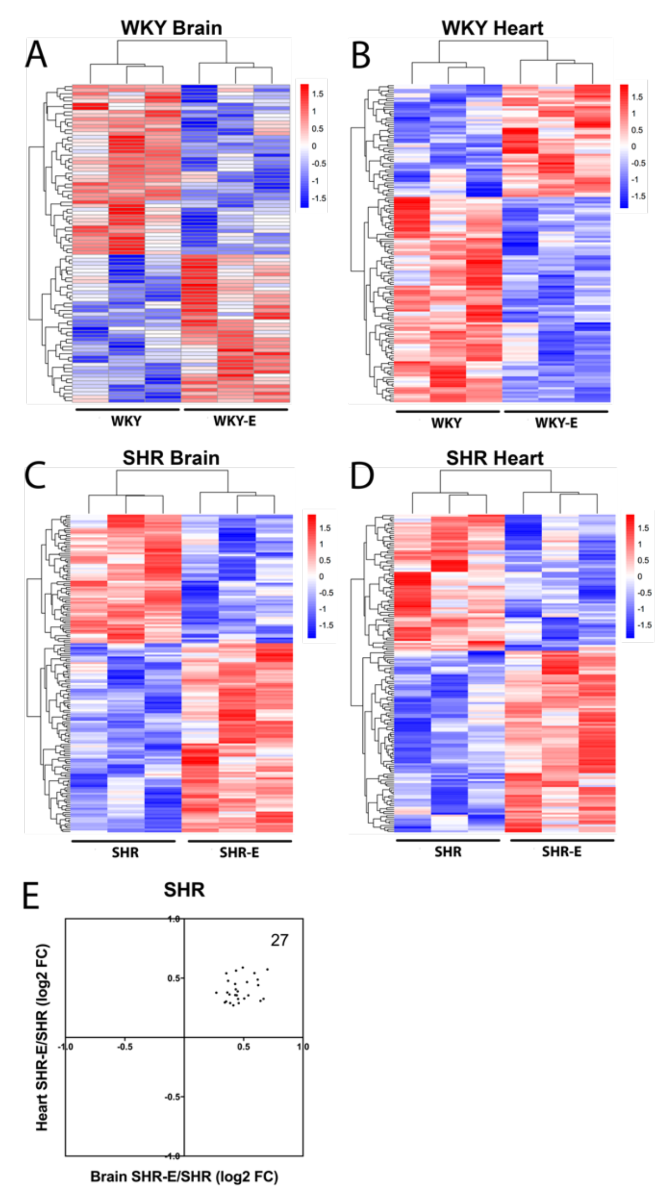
In a new window | Download PPT
Figure 5: Vasculome heatmap of exercise-induced DEGs in (A) brain and (B) heart of WKY, and in (C) brain and (D) heart of SHR. (E) Distribution of fold-change directions in exercise-induced common DEGs in brain and heart vasculomes of SHR.
Table 3: Effects of exercise on WKY and SHR vasculomes.
(A) Numbers of significantly changed DEGs and pathways:
|
DEG |
Reactome |
||||||
|
Exercise |
WKY |
SHR |
Common in rats |
|
WKY |
SHR |
Common in rats |
|
Brain |
88 |
168 |
2 |
41 |
107 |
15 |
|
|
Heart |
147 |
161 |
9 |
64 |
51 |
13 |
|
|
Common in organs |
5 |
27 |
0 |
8 |
33 |
5 |
|
(B) List of commonly changed Reactome pathways:
|
WKY, Brain |
WKY, Heart |
SHR, Brain |
SHR, Heart |
|
||||||||
|
Reactome by exercise |
size |
NES |
p |
NES |
p |
NES |
p |
NES |
p |
|||
|
Potassium channels |
93 |
1.90 |
0.000 |
1.43 |
0.017 |
-1.60 |
0.005 |
-1.65 |
0.003 |
|||
|
G alpha (i) signaling events |
166 |
-1.36 |
0.016 |
1.66 |
0.000 |
-1.47 |
0.003 |
-1.40 |
0.011 |
|||
|
GPCR ligand binding |
352 |
-1.47 |
0.000 |
1.63 |
0.000 |
-1.59 |
0.000 |
-1.72 |
0.000 |
|||
|
Peptide ligand binding receptors |
156 |
-1.74 |
0.000 |
1.62 |
0.000 |
-1.41 |
0.017 |
-1.54 |
0.001 |
|||
|
Class A/1 Rhodopsin-like receptors |
259 |
-1.77 |
0.000 |
1.64 |
0.000 |
-1.49 |
0.003 |
-1.61 |
0.000 |
|||
Patterns of response were also detected when looking at pathways affected by exercise in brains and hearts of both WKY and SHR (Figure 6A-D). Reactome categories of these exercise effects broadly included immune response, metabolism, signal transduction, cell cycle, and transport of small molecules. Once again, exercise appeared to modify the hypertensive vasculome in a consistent way. There were 33 common Reactome pathways in brains and hearts of exercised SHR. In 30 of them, exercise moved these pathways in the same direction (upregulate or downregulate) (Figure 6E).
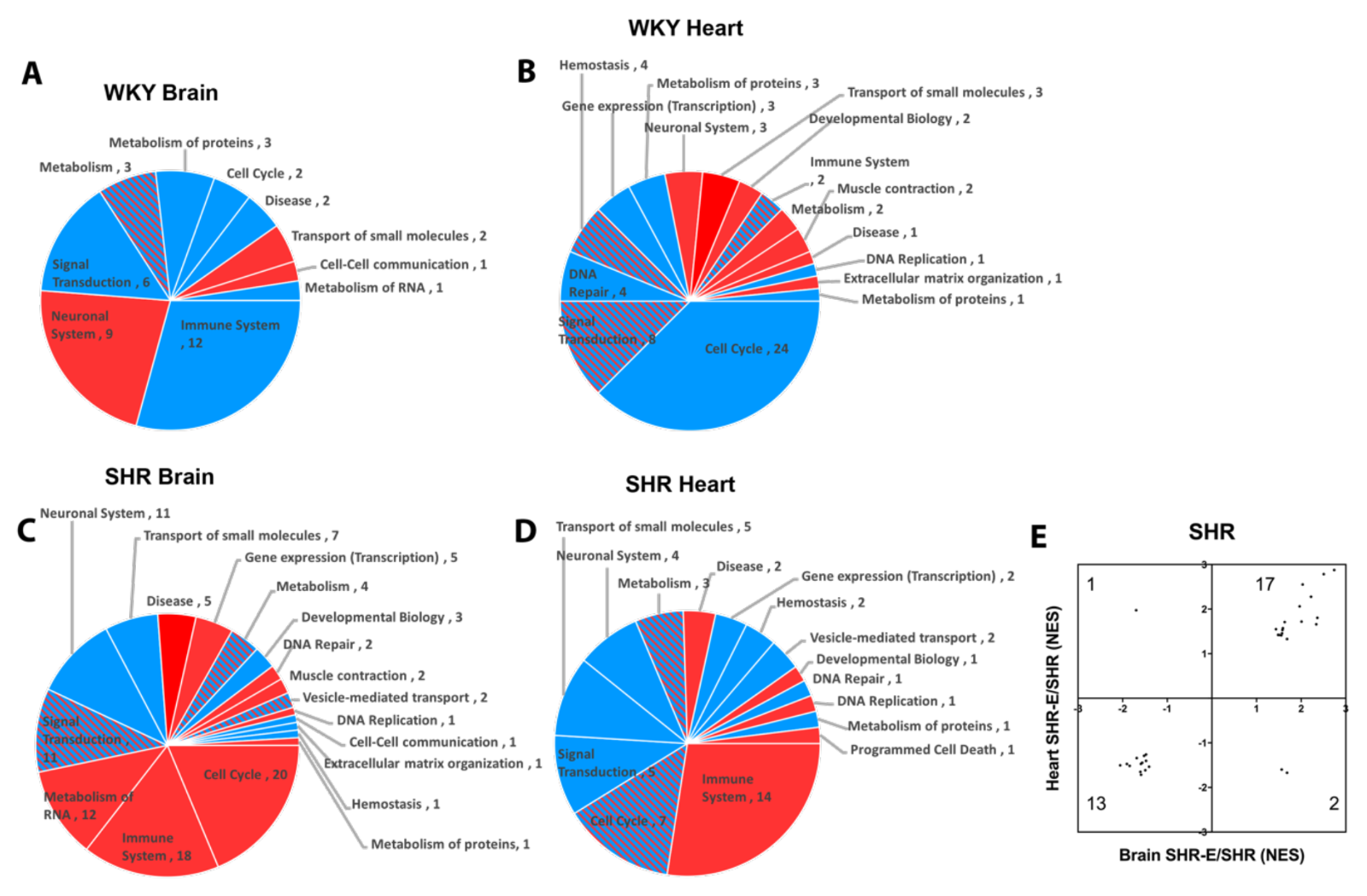
In a new window | Download PPT
Figure 6: Pie chart of exercise-induced Reactome pathways in (A) brain and (B) heart of WKY, and in (C) brain (C) and (D) heart of SHR; red for upregulation, blue for downregulation, and shaded for mixed directions of change. (E) Distribution of change directions in exercise-induced common Reactome pathways in brain and heart vasculomes of SHR.
Finally, focusing only on exercise-affected pathways that were present in both brains and hearts, and present in both WKY and SHR resulted in a subset of 5 potentially conserved pathways (Table 3). Three of these pathways (potassium channels, G-protein coupled receptor (GPCR) ligand binding, and class A/1 rhodopsin-like receptors) overlapped with the core subset of pathways that were affected by hypertension in rat and mouse vasculomes (Table 2).
Renormalization of the hypertensive vasculome by exercise
Finally, based on the data, we asked whether exercise may “renormalize” the hypertensive vasculome? To answer this question, we defined genes that were significantly altered by hypertension, i.e. comparing SHR vs WKY vasculomes. Plotting hypertensive DEGs in sedentary SHR vs sedentary WKY demonstrated that, as expected, some genes were upregulated (above unity line) and some genes were downregulated (below unity line) in both brain and heart vasculomes (Figure 7A-B). Then we assessed the effects of exercise by comparing the same genes in exercised SHR vs sedentary WKY. Replotting these DEGs showed that exercise appeared to partly renormalize the hypertensive vasculomes in both brain and heart, i.e. the gap around the unity line is filled back in (Figure 7C-D).
To further assess the details of this renormalization phenomenon, we focused on vasculome responses that were significantly affected by both hypertension (sedentary SHR vs sedentary WKY) and exercise (exercised SHR vs sedentary SHR). This subset of the vasculome comprised 78 genes in the brain and 72 genes in the heart. Heatmaps demonstrated that exercise tended to shift the hypertensive vasculome “back closer to normal”, i.e. colors were more similar in exercised SHR and sedentary WKY (Figure 7E-F). A few representative “renormalized genes” are graphed in Figure 7G. For example, Snca (synuclein alpha) is a major component of Lewy body and Lewy neurite, closely related with neurodegeneration disease, including PD and AD (Goedert, 2015; Siddiqui et al., 2016). Snca is upregulated in the hypertensive brain vasculome of SHR, and this is ameliorated by exercise. Kcnq5 protein (potassium voltage-gated channel subfamily Q member 5) regulates intrinsic myogenicity and vasodilation in cerebral and coronary arteries. It is upregulated in hypertensive brain vasculome then decreased by exercise. Similarly, the cation transporter regulator Chac2 is upregulated in the SHR brain vasculome and decreased by exercise. Renormalization may also occur for downregulated genes. The TNF-inducible endothelial factor ApoL9a is decreased in SHR brain and increased in the exercised SHR brain. Similarly, hypertensive reductions in pro-inflammatory Rsad2 and Ccl2 are also partially ameliorated by exercise. Altogether, over 85% of these hypertensive genes (74 out of 78 in brain, and 63 out of 72 in heart) had opposite directions (i.e. partly renormalized) when comparing the effect of hypertension versus the effect of exercise (Figure 7H-I).
Pathway analysis confirmed the potential renormalization effect of exercise. Many hypertensive pathways were affected by exercise, including immune function, signal transduction, and metabolism (Figure 7J-K). After exercise, over two thirds of these pathways appeared to move in opposite directions (26 out of 35 in brain, and 10 out of 14 in heart). In the brain, pathways for immune system including interferon induction and signaling, and smooth muscle contraction were all downregulated by hypertension then renormalized by exercise; whereas small molecule transport, metabolite biosynthesis, and some neurotransmitter related pathways were all upregulated by hypertension then renormalized with exercise. In the heart, pathways for the immune system including interferon signaling and cytokine signaling were downregulated by hypertension then renormalized with exercise; whereas pathways for small molecule transport, and signal transduction including GPCR and Rhodoposin like receptors were upregulated by hypertension then renormalized with exercise. However, not all pathways were equally affected; GPCR-related pathways induced by hypertension in the brain vasculome were not renormalized by exercise.
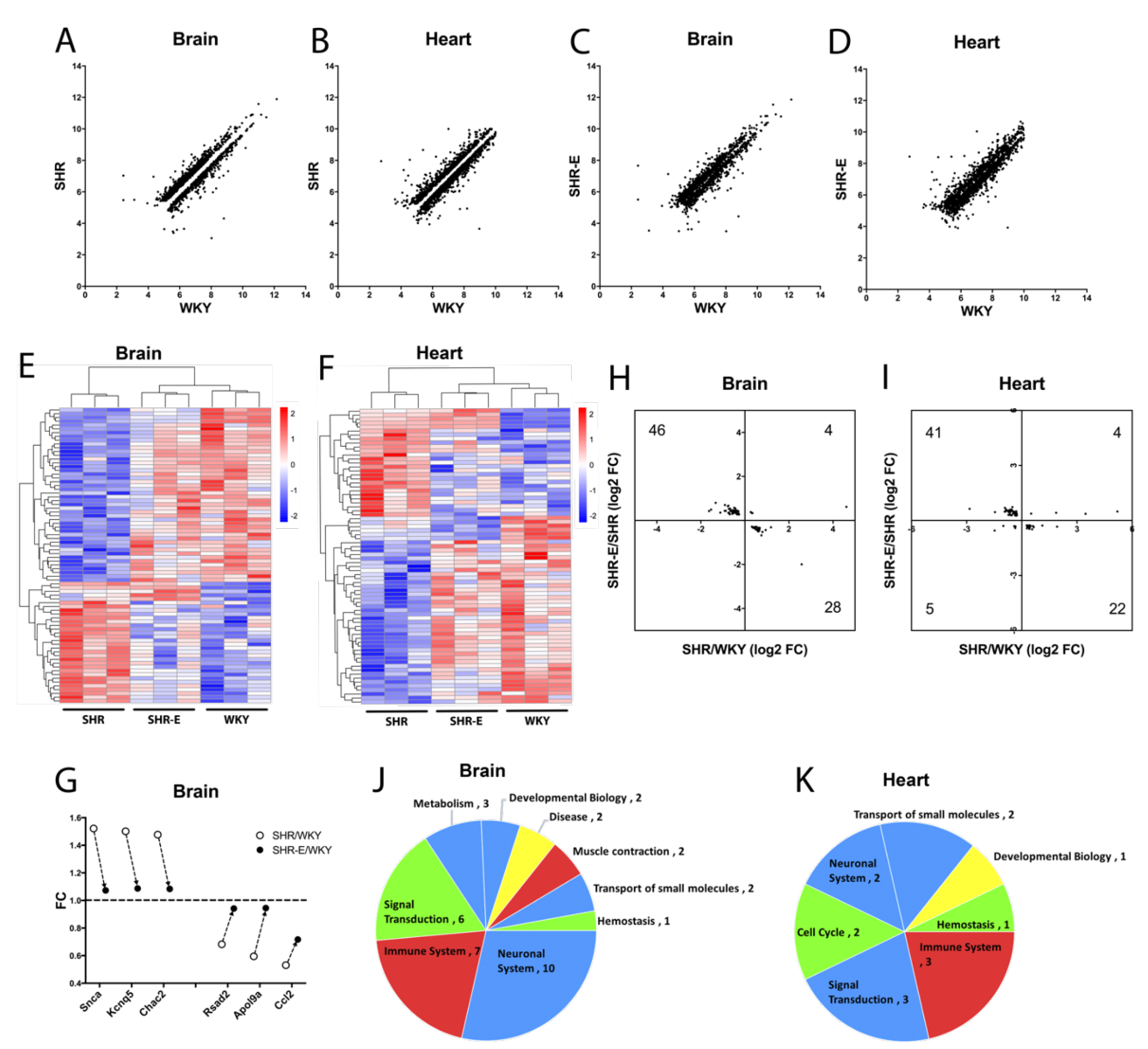
In a new window | Download PPT
Figure 7: Distribution of hypertension-induced DEGs (sedentary SHR vs sedentary WKY) in (A) brain and (B) heart vasculomes. Only significantly up- or downregulated genes are plotted so there is a gap around the unity line. Effects of exercise on hypertension-induced DEGs (exercised SHR-E vs sedentary WKY) in (C) brain and (D) heart. The gap is partly filled in suggesting renormalization. Heatmap of commonly affected hypertension DEGs in (E) brain and (F) heart of three groups of rats (sedentary WKY, sedentary SHR, and exercised SHR-E). (G) Examples of specific genes changed with hypertension and then renormalized by exercise in SHR brain vasculome. Distribution of fold-change directions in commonly affected DEGs by hypertension (SHR vs WKY) and exercise (SHR-E vs SHR) in (H) brain and (I) heart. Pie chart of commonly affected Reactome pathways by hypertension and exercise in (J) brain and (K) heart of SHR. Renormalized pathways are labeled in red and blue; red for downregulated by hypertension but upregulated by exercise, blue for upregulated by hypertension but downregulated by exercise. Non-normalized pathways are labeled in green and yellow; green for downregulated by both hypertension and exercise, yellow for upregulated by both hypertension and exercise.
Discussion
In the current study we showed that (i) brain and heart vasculomes were significantly different in hypertensive SHR compared to normotensive WKY, and (ii) treadmill running exercise partly renormalized the hypertensive brain and heart vasculomes in SHR. Common responses to hypertension were detected when comparing the present findings in SHR with previously published vasculomes from hypertensive mouse models, suggesting that overlapping mechanisms may exist across species for potential translational relevance. Both common and unique pathways were detected in the brain and heart after exercise, suggesting the importance of conserved mechanisms as well as organ-specific responses. These findings support the utility of the vasculome as a conceptual framework for generating and testing hypotheses to dissect mechanisms in neurovascular and cardiovascular disease. Our results also support the idea that the beneficial effects of exercise should be viewed as a form of “conditioning medicine” and hence, may be a powerful probe for exploring novel targets and therapeutic strategies in the vasculome.
An important caveat with this proof-of-principle study is that we did not define causal and/or physiological mechanisms. How treadmill running alters gene expression in the brain and heart microvessels remains to be investigated, although it is likely that multifactorial direct and indirect mechanisms will be involved. In our experiment, exercise did not appear to change blood pressure. Mixed results have been reported in the literature. Some studies showed that blood pressure in SHR was reduced after several weeks of exercise (Boone and Corry, 1996; de Andrade et al., 2015; Garcia-Pinto et al., 2011; Masson et al., 2014; Moraes-Teixeira Jde et al., 2010; Passos et al., 2016), while others showed that exercise did not change blood pressure (Kohzuki et al., 2001; Yoshida et al., 2003; Ziada, 2009). In fact, two studies surprisingly suggest that blood pressure was increased after weeks of exercise (Pagan et al., 2015; Ziada et al., 2005). These different results may be related to different experimental parameters, especially related to the intensity of exercise. Low-intensity (30% of maximal aerobic velocity at 9-10 m/min or 55% V O2 max at 16-20 m/min) but not moderate-intensity exercise (60% of maximal aerobic velocity at 18–20 m/min or 85% V O2max at 25-30 m/min) lowered blood pressure in SHR (Sun et al., 2008; Veras-Silva et al., 1997). Differences may also depend on the use of voluntary or forced exercise. Systolic blood pressure increased by 21 mmHg in voluntarily exercised SHR versus 34 mmHg in forced exercised SHR (Higuchi et al., 1985). Central control mechanisms may also play a role, since the nucleus tractus solitarii (NTS) controls blood pressure during exercise, and the transcriptome of NTS samples suggest that antihypertensive effects of exercise in SHR may be related to inflammatory responses in this brain region (Waki et al., 2013). Finally, although functional endpoints were not emphasized in this exploratory study, some potential changes in rat behavior may be present. For example, some studies propose that SHRs may be hyperactive and so might be used as an animal model for attention deficit disorders (Bayless et al., 2015; Sagvolden et al., 2005). It is interesting to note that exercise appeared to slightly decrease entry number in the Y maze, and this may indirectly reflect an amelioration of hyperactivity. Ultimately, the challenge is to find mechanisms to link cause (exercise-mediated vasculome responses) with effect (protection against injury or disease, and improvements in organ function).
In general, the beneficial effects of exercise on blood vessels or endothelial cells have been widely reported (Trigiani and Hamel, 2017). Exercise increases blood flow velocity, and reduces shear stress in vascular walls (Dolan et al., 2013). Some of these effects may be structural since regular exercise may reduce vascular stiffness and collagen deposition (Roque et al., 2013). Flow may also be mediated by effects on thrombosis, which was significantly decreased by exercise in stroke-prone spontaneously hypertensive rats (Sasaki et al., 2004). In SHR rats, the impairment of acetylcholine-induced vasorelaxation could be improved by exercise through several mechanisms, including the upregulation of nitric oxide synthase or reduction of superoxide production (Guerrero et al., 2013; Yang et al., 2011). Endothelial dysfunction and oxidative stress along with disruptions in baroreflex in SHR rats may all be improved by exercise (Bertagnolli et al., 2006). Vasodilator-stimulated phosphoprotein (VASP), which is required for cytoskeleton organization in endothelial cells, was reduced by hypertension, while exercise normalized its expression and phosphorylation levels in cerebral endothelial cells (Arlier et al., 2015). Once again, the intensity of exercise may be important: moderate-intensity exercise induced beneficial effects on endothelial-dependent vasorelaxation, but high-intensity exercise did not (Battault et al., 2016). Exercise training also improved large and numerous cytoplasmatic vacuoles, fragmented inner elastic lamina and scarce elastin and fibrillin in endothelial cells, which is related to endothelial dysfunction in SHR (Moraes-Teixeira Jde et al., 2010). Exercise upregulated BDNF in cardiac and cerebral endothelial cells, which was decreased in SHR compared with WKY rats (Monnier et al., 2017a; Monnier et al., 2017b; Prigent-Tessier et al., 2013). Vascular effects of exercise can significantly affect the response of tissue to injury. For example, exercise performed before stroke may act as a form of pre-conditioning and reduce infarcts in rodent models of cerebral ischemia (Ding et al., 2004; Ding et al., 2006; Liebelt et al., 2010). However, these effects are complex and time-dependent so that forced exercise too soon post-stroke may actually worsen outcomes (Dornbos and Ding, 2012; Li et al., 2017). Altogether, exercise should induce a wide range of complex vascular actions that may be difficult to test on a singular pathway by pathway basis. In this regard, a vasculome analysis may offer a systematic way to dissect this phenomenon, and the present findings suggest that such an approach may be feasible.
Because the vasculome represents a large database, it may be helpful for initial analyses to focus on common responses. Across both brains and hearts in hypertensive SHR rats (the present study) and hypertensive BPH mice (Guo et al., 2018), 5 common genes were identified: Death-associated protein kinase 1 (DAPK1), DNA ligase 4 (Lig4), SET domain containing lysine methyltransferase 7 (Setd7), tripartite motif-containing 16 (Trim16), and UDP-glucose glycoprotein glucosyltransferase 2 (Uggt2). DAPK1, a Ca2+/calmodulin-dependent serine/threonine protein kinase, is a regulator of apoptosis and autophagy (Singh et al., 2016). DAPK1 may play an important role in stroke-induced cell death (Wang et al., 2017; White et al., 2012). The activation of DAPK1 is also responsible for selective degeneration of CA1 synapses in Alzheimer’s disease (Shu et al., 2016). LIG4 is involved in DNA-double-strand break repair mechanisms (Helleday et al., 2007), and is essential for lymphogenesis and neurogenesis during development (Frank et al., 2000; Helleday et al., 2007). Setd7 regulates cardiomyocyte differentiation through transcriptional activation, cell cycle regulation, and ROS signaling (Chen et al., 2016; He et al., 2015; Lee et al., 2018). Trim16 is also a regulator of cell proliferation and cell death, which may control the expression of interferon beta 1 (Marshall et al., 2010; Sutton et al., 2014). Uggt2 is an important component of glycoprotein folding cycle that is essential for recognizing and modifying misfolded proteins in the endoplasmic reticulum (Takeda et al., 2014). Taken together, it is reasonable to expect that these 5 genes should play broad roles in maintaining organ homeostasis during normal function so perturbations induced by hypertension may promote dysfunction during injury or disease. What was somewhat surprising was that none of these genes were robustly affected by exercise in SHR. The meaning of this lack of effect is unclear but it may imply that beneficial actions of exercise may involve recruitment of alternate pathways.
In terms of GSEA analysis, 3 common pathways were perturbed by hypertension in SHRs and then renormalized by running exercise: potassium channels, GPCR ligand binding, and class A/1 rhodopsin-like receptors. Potassium channels form a big family of mediators and participate in many vital biological functions (Tian et al., 2014), including the contribution of endothelial potassium channels to blood pressure regulation (Haddy et al., 2006; Wandall-Frostholm et al., 2014). GPCR is the largest receptor superfamily in the human genome, and rhodopsin-like receptors (class A/1) are the largest and the best studied group of GPCRs by far. This large group can be further classified into 19 subgroups, including chemokine receptors and angiotensin receptors (Joost and Methner, 2002), all of which are known to be important in neurovascular and cardiovascular disease (Brinks and Eckhart, 2010; Wang et al., 2018). Potassium channels were renormalized in both brain and heart of SHR, while GPCR ligand binding and Rhodopsin-like receptors were renormalized only in heart of SHR. Further dissection of these pathways and gene networks may reveal novel targets underlying the broad effects of exercise in the vasculome.
There is significant overlap between rodent vasculomes and human disease genes (Guo et al., 2012). Hence, it is reasonable to ask whether and how exercise-mediated vasculome responses may also be linked to human databases? For example, it has been shown that exercise reduced Snca levels in aged humans (Daniele et al., 2018). Similarly here, we first saw an increase in Snca in the brain vasculome of SHR, and then exercise reduced its expression. A human hypertension GWAS gene CD36 was also found to be significantly changed in the heart of SHR. CD36, a fatty acid translocase, was identified as an insulin-resistance gene causing defective fatty acid and glucose metabolism in SHR (Aitman et al., 1999). Deficient renal expression of CD36 has been reported as a genetically determined risk for the development of hypertension in SHR (Pravenec et al., 2008). But in this case, exercise in SHR did not reverse hypertension-induced changes in CD36. Additional and more detailed analyses are warranted to mine the entire exercise-vasculome database for additional links to human risk factors and disease.
In conclusion, this proof-of-principle study suggests that exercise may partly renormalize the hypertensive vasculome in brains and hearts of SHR rats. Further studies are warranted to pursue causal mechanisms and leverage this vasculome database for the pursuit of novel targets and biomarkers in neurovascular and cardiovascular disease.
Acknowledgments
Supported in part by grants from NIH, the Rappaport Foundation and China National Key R&D Program (2017YFC1308401).
References
Jing Lan*
1Department of Neurology and China-America Institute of Neuroscience, Xuanwu Hospital, Capital Medical University, Beijing, China.
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Shuzhen Guo*
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Jingfei Shi
1Department of Neurology and China-America Institute of Neuroscience, Xuanwu Hospital, Capital Medical University, Beijing, China.
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Elga Esposito
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Emiri T. Mandeville
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Wenjun Deng
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
3Clinical Proteomics Research Center, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Christiane D. Wrann
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
4Cardiovascular Research Center, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
MingMing Ning
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
3Clinical Proteomics Research Center, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Xunming Ji
1Department of Neurology and China-America Institute of Neuroscience, Xuanwu Hospital, Capital Medical University, Beijing, China.
Eng H. Lo
2Neuroprotection Research Laboratories, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
3Clinical Proteomics Research Center, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Corresponding author:
Eng H. Lo
Email: Lo@helix.mgh.harvard.edu
and
Xunming Ji
Email: jixunming@vip.163.com
*These authors contributed equally to this work.
Dr. Perez-Pinzonn served as handling editor for this article.

In a new window | Download PPT
Figure 1: Comparison of different cell type markers between endothelial cell samples and their original organ tissue in (A) brain or (B) heart. (C) Comparison of some differentially expressed genes between RT-PCR and microarray results.

In a new window | Download PPT
Figure 2: (A) Principal component analysis of vasculomes from 4 groups. Heatmap of hypertension-induced DEGs in SHR (B) brain and (C) heart. (D) Distribution of fold-change directions for common DEGs in brain and heart of SHR. Pie chart of Reactome pathways induced by hypertension in (E) SHR brain and (F) SHR heart; red for upregulation, blue for downregulation, and shaded for mixed directions of change. (G) Distribution of change directions for common Reactome pathways in brain and heart of SHR.

In a new window | Download PPT
Figure 3: Distribution of fold-change directions for common hypertension DEGs in SHR-vs-WKY and BPH-vs-BPN in (A) brain and (B) heart. Distribution of change directions for hypertension-induced common Reactome pathways in SHR-vs-WKY and BPH-vs-BPN in (C) brain and (D) heart.

In a new window | Download PPT
Figure 4: Body weight during the process of exercise in (A) normotensive WKY and (B) hypertensive SHR. Blood pressure during the process of exercise in (C) normotensive WKY and (D) hypertensive SHR. Total entry numbers in Y-maze test for (E) WKY and (F) SHR. Ratio of correct entry (percentage) in Y-maze for (G) WKY and (H) SHR. * for p < 0.05 and ** for p < 0.01.

In a new window | Download PPT
Figure 5: Vasculome heatmap of exercise-induced DEGs in (A) brain and (B) heart of WKY, and in (C) brain and (D) heart of SHR. (E) Distribution of fold-change directions in exercise-induced common DEGs in brain and heart vasculomes of SHR.

In a new window | Download PPT
Figure 6: Pie chart of exercise-induced Reactome pathways in (A) brain and (B) heart of WKY, and in (C) brain (C) and (D) heart of SHR; red for upregulation, blue for downregulation, and shaded for mixed directions of change. (E) Distribution of change directions in exercise-induced common Reactome pathways in brain and heart vasculomes of SHR.

In a new window | Download PPT
Figure 7: Distribution of hypertension-induced DEGs (sedentary SHR vs sedentary WKY) in (A) brain and (B) heart vasculomes. Only significantly up- or downregulated genes are plotted so there is a gap around the unity line. Effects of exercise on hypertension-induced DEGs (exercised SHR-E vs sedentary WKY) in (C) brain and (D) heart. The gap is partly filled in suggesting renormalization. Heatmap of commonly affected hypertension DEGs in (E) brain and (F) heart of three groups of rats (sedentary WKY, sedentary SHR, and exercised SHR-E). (G) Examples of specific genes changed with hypertension and then renormalized by exercise in SHR brain vasculome. Distribution of fold-change directions in commonly affected DEGs by hypertension (SHR vs WKY) and exercise (SHR-E vs SHR) in (H) brain and (I) heart. Pie chart of commonly affected Reactome pathways by hypertension and exercise in (J) brain and (K) heart of SHR. Renormalized pathways are labeled in red and blue; red for downregulated by hypertension but upregulated by exercise, blue for upregulated by hypertension but downregulated by exercise. Non-normalized pathways are labeled in green and yellow; green for downregulated by both hypertension and exercise, yellow for upregulated by both hypertension and exercise.
Table 1: Common Reactome pathways changed by hypertension in brain and heart of SHR vasculomes.
|
Brain |
Heart |
|||||
|
Reactome, SHR/WKY |
size |
NES |
p |
NES |
p |
|
|
Neuronal system |
260 |
2.05 |
0.000 |
1.37 |
0.016 |
|
|
Potassium channels |
93 |
2.04 |
0.000 |
1.49 |
0.014 |
|
|
Interaction between L1 and ankyrins |
21 |
1.69 |
0.014 |
1.58 |
0.027 |
|
|
CREB phosphorylation through the activation of Ras |
23 |
1.68 |
0.023 |
1.77 |
0.014 |
|
|
G alpha (S) signaling events |
112 |
-1.35 |
0.041 |
-1.43 |
0.018 |
|
|
Signaling by ILs |
100 |
-1.62 |
0.005 |
-1.39 |
0.036 |
|
|
Smooth muscle contraction |
15 |
-1.84 |
0.009 |
-1.63 |
0.032 |
|
|
Cytokine signaling in immune system |
218 |
-1.93 |
0.000 |
-1.63 |
0.000 |
|
|
Interferon signaling |
117 |
-2.06 |
0.000 |
-1.54 |
0.004 |
|
|
Interferon gamma signaling |
48 |
-2.31 |
0.000 |
-1.71 |
0.004 |
|
|
Cholesterol biosynthesis |
21 |
1.62 |
0.029 |
-2.04 |
0.000 |
|
|
Mitotic G2 - G2/M phases |
68 |
1.39 |
0.045 |
-1.53 |
0.023 |
|
|
Cell cycle mitotic |
275 |
1.26 |
0.041 |
-2.00 |
0.000 |
|
|
GPCR lignad binding |
352 |
-1.53 |
0.000 |
1.37 |
0.004 |
|
|
Complement cascade |
23 |
-1.60 |
0.044 |
1.76 |
0.008 |
|
|
Class A/1 Rhodopsin-like receptors |
259 |
-1.67 |
0.000 |
1.49 |
0.006 |
|
|
Peptide ligand binding receptors |
156 |
-1.88 |
0.000 |
1.47 |
0.006 |
|
Table 2: Comparisons between hypertensive rat SHR and hypertensive mouse BPH.
(A) Numbers of significantly changed DEGs and pathways:
|
DEG |
Reactome |
||||||
|
Hypertension |
SHR |
BPH |
Common in species |
|
SHR |
BPH |
Common in species |
|
Brain |
1318 |
1206 |
79 |
82 |
25 |
13 |
|
|
Heart |
1422 |
978 |
108 |
77 |
44 |
9 |
|
|
Common in organs |
323 |
231 |
5 |
17 |
9 |
4 |
|
(B) List of commonly changed DEGs:
|
Rat |
SHR, Brain |
SHR, Heart |
Mouse |
BPH, Brain |
BPH, Heart |
|||||
|
Symbol |
Gene ID |
FC |
p |
FC |
p |
Gene ID |
FC |
p |
FC |
p |
|
Dapk1 |
306722 |
1.29 |
0.041 |
-1.21 |
0.013 |
69635 |
-1.93 |
0.032 |
-1.74 |
0.002 |
|
Lig4 |
290907 |
2.82 |
0.000 |
1.41 |
0.033 |
319583 |
-10.76 |
0.001 |
-4.06 |
0.005 |
|
Setd7 |
689954 |
1.25 |
0.023 |
1.22 |
0.008 |
73251 |
2.11 |
0.002 |
1.36 |
0.004 |
|
Trim16 |
303214 |
2.02 |
0.000 |
1.88 |
0.000 |
94092 |
3.31 |
0.002 |
3.96 |
0.000 |
|
Uggt2 |
361091 |
1.62 |
0.005 |
2.40 |
0.000 |
64435 |
-1.44 |
0.007 |
-1.61 |
0.002 |
(C) List of commonly changed Reactome pathways:
|
Rat |
SHR, Brain |
SHR, Heart |
Mouse |
BPH, Brain |
BPH, Heart |
|||||
|
Reactome |
size |
NES |
p |
NES |
p |
size |
NES |
p |
NES |
p |
|
Potassium channels |
93 |
2.04 |
0.000 |
1.49 |
0.014 |
58 |
1.42 |
0.031 |
1.95 |
0.001 |
|
GPCR ligand binding |
352 |
-1.53 |
0.000 |
1.37 |
0.004 |
144 |
-1.49 |
0.002 |
1.47 |
0.002 |
|
Class A/1 Rhodopsin-like receptors |
259 |
-1.67 |
0.000 |
1.49 |
0.006 |
90 |
-1.55 |
0.005 |
1.47 |
0.013 |
|
Cytokine signaling in immune system |
218 |
-1.93 |
0.000 |
-1.63 |
0.000 |
178 |
-1.45 |
0.004 |
1.32 |
0.032 |
Table 3: Effects of exercise on WKY and SHR vasculomes.
(A) Numbers of significantly changed DEGs and pathways:
|
DEG |
Reactome |
||||||
|
Exercise |
WKY |
SHR |
Common in rats |
|
WKY |
SHR |
Common in rats |
|
Brain |
88 |
168 |
2 |
41 |
107 |
15 |
|
|
Heart |
147 |
161 |
9 |
64 |
51 |
13 |
|
|
Common in organs |
5 |
27 |
0 |
8 |
33 |
5 |
|
(B) List of commonly changed Reactome pathways:
|
WKY, Brain |
WKY, Heart |
SHR, Brain |
SHR, Heart |
|
||||||||
|
Reactome by exercise |
size |
NES |
p |
NES |
p |
NES |
p |
NES |
p |
|||
|
Potassium channels |
93 |
1.90 |
0.000 |
1.43 |
0.017 |
-1.60 |
0.005 |
-1.65 |
0.003 |
|||
|
G alpha (i) signaling events |
166 |
-1.36 |
0.016 |
1.66 |
0.000 |
-1.47 |
0.003 |
-1.40 |
0.011 |
|||
|
GPCR ligand binding |
352 |
-1.47 |
0.000 |
1.63 |
0.000 |
-1.59 |
0.000 |
-1.72 |
0.000 |
|||
|
Peptide ligand binding receptors |
156 |
-1.74 |
0.000 |
1.62 |
0.000 |
-1.41 |
0.017 |
-1.54 |
0.001 |
|||
|
Class A/1 Rhodopsin-like receptors |
259 |
-1.77 |
0.000 |
1.64 |
0.000 |
-1.49 |
0.003 |
-1.61 |
0.000 |
|||
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 9069 | 21 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA