Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Diabetes mellitus worsens outcomes in tPA thrombolytic stroke therapy
Time:2019-03-02
Number:14777
Yinghua Jiang1,2, Ning Liu2,3, Zeyuan Cao2, Lena Huang2, Zhanyang Yu2, Qiuchen Zhao2,4, Fang Zhang2,5, Ming-Ming Ning2, Klaus van Leyen2, Eng H. Lo2, Xiaoying Wang2
Author Affiliations


- 1Department of Neurosurgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
- 2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
- 3The Third Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
- 4Department of Neurology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, Jiangsu 210008, China.
- 5Department of Neurology, Tianjin Neurological Institute, Tianjin Medical University General Hospital, Tianjin 300052, China.
Conditioning Medicine, 2019. 2(1):2-9.
Abstract
The demonstrated benefit of tissue type plasminogen activator (tPA) thrombolytic therapy has been a landmark achievement in stroke therapy. However, not all patients respond equally, and some patients have worse outcomes. In particular, diabetes mellitus is recognized as a clinically important vascular co-morbidity that leads to lower recanalization rates and increased risk of hemorrhagic transformation. In this mini-review, we summarize recent advances in the understanding of the underlying mechanisms involved in worse stroke outcome in diabetic patients. Potential pathological factors that relate to suboptimal recanalization include higher plasma plasminogen activator inhibitor-1 levels, diabetic atherogenic vascular damage, glycation of the tPA receptor annexin A2, alterations in fibrin clot density, and impaired collaterals. Factors that may contribute to hemorrhagic transformation include hyperglycemia, vascular oxidative stress, inflammation, tPA neurovascular toxicity, and dysfunction in extracellular proteolysis balance. A better understanding of these complex pathways may eventually lead to novel ways of counteracting the negative effects of diabetes in ischemic stroke.
Keywords: ischemic stroke, tissue-type plasminogen activator, diabetes mellitus, hyperglycemia, recanalization rate, intracerebral hemorrhage
Abstract
The demonstrated benefit of tissue type plasminogen activator (tPA) thrombolytic therapy has been a landmark achievement in stroke therapy. However, not all patients respond equally, and some patients have worse outcomes. In particular, diabetes mellitus is recognized as a clinically important vascular co-morbidity that leads to lower recanalization rates and increased risk of hemorrhagic transformation. In this mini-review, we summarize recent advances in the understanding of the underlying mechanisms involved in worse stroke outcome in diabetic patients. Potential pathological factors that relate to suboptimal recanalization include higher plasma plasminogen activator inhibitor-1 levels, diabetic atherogenic vascular damage, glycation of the tPA receptor annexin A2, alterations in fibrin clot density, and impaired collaterals. Factors that may contribute to hemorrhagic transformation include hyperglycemia, vascular oxidative stress, inflammation, tPA neurovascular toxicity, and dysfunction in extracellular proteolysis balance. A better understanding of these complex pathways may eventually lead to novel ways of counteracting the negative effects of diabetes in ischemic stroke.
Keywords: ischemic stroke, tissue-type plasminogen activator, diabetes mellitus, hyperglycemia, recanalization rate, intracerebral hemorrhage
Tissue plasminogen activator (tPA): thrombolytic stroke therapy and its limitations
Ischemic stroke is a cerebrovascular event. Despite enormous research efforts that include many clinical trials, intravenous administration of recombinant tPA remains the only FDA-approved treatment for ischemic stroke, and the most beneficial proven intervention for emergency treatment of ischemic stroke (Chapman et al., 2014; Whiteley et al., 2014). tPA thrombolytic stroke therapy is based on the "recanalization hypothesis," which posits that reopening of occluded vessels by lysing the clot will improve clinical outcome in acute ischemic stroke (AIS) through regional reperfusion and salvaging threatened tissues (Whiteley et al., 2014). Recanalization is an important predictor of stroke outcome after thrombolysis. The demonstrated clinical benefit of thrombolytic therapy has been a landmark discovery in the treatment of AIS. However, there are disadvantages to the use of thrombolytic therapy including lower thrombolytic perfusion rate, short therapeutic treatment time window, and risk of hemorrhagic transformation (Alexandrov and Grotta, 2002; Rubiera et al., 2005; Bambauer et al., 2006; Weintraub, 2006). Overcoming these disadvantages and making tPA work more effectively is a priority that will aid in advancing AIS treatment (Davalos, 2005; Thomassen and Bakke, 2007). Although other thrombolytic agents are being tested, none have been shown to be an effective replacement for tPA (Adams et al., 2007). Importantly, exogenous tPA may worsen ischemia-induced blood brain-barrier (BBB) disruption, elevate the risk of symptomatic intracranial hemorrhage, and in part, reduce the therapeutic time window (Chapman et al., 2014). Recent clinical investigations have indicated potential opportunities to improve tPA therapy. For instance, European ECASS III trial showed that intravenous tPA given up to 4.5 hr after symptom onset improved clinical outcomes (Cronin, 2010). In the Echoplanar Imaging Thrombolytic Evaluation trial, thrombolysis 4.5-6 hr after stroke onset reduced infarct growth and increased the rate of reperfusion, which was associated with good neurological and functional outcome (Picanco et al., 2014). Moreover, recent trials point to potential avenues to improve patient access by imaging-based patient selection, and to facilitate rapid and complete reperfusion of the penumbra (Manning et al., 2014). One such avenue includes endovascular thrombectomy performed from 6 to 24 hrs after stroke onset (Nogueira et al., 2018). This review will only focus on intravenous tPA thrombolytic reperfusion therapy (IV tPA).
Although imaging-based patient selection may help to identify more ischemic stroke patients who are candidates for IV tPA, IV tPA is still associated with increased intracranial hemorrhagic transformation, which remains the most threatened complication for thrombolytic stroke therapy (Harsany et al., 2014). There are a number of risk factors associated with tPA stroke therapy-mediated intracerebral hemorrhagic transformation, including diabetes mellitus (DM), post-stroke hyperglycemia, older age, larger infarct, and high blood pressure (Miller et al., 2011; Faigle et al., 2014; Shrestha et al., 2014). In this mini review, we focus on the role and mechanism(s) of DM as a risk factor for the lower recanalization rate and intracerebral hemorrhagic transformation after tPA thrombolytic stroke therapy. Although the molecular mechanisms underlying DM-related complications after stroke remain to be further elucidated, a better understanding of these complex pathways may eventually lead to novel ways of counteracting the negative effects of diabetes in stroke (Fan et al., 2014; Shrestha et al., 2014; Li et al., 2017).
DM in stroke clinical epidemiology
DM is a major risk factor for cardiovascular diseases, including stroke. Globally, stroke mortality rates have fallen, but stroke incidence and its sequelae have significantly increased over the last three decades (Hill, 2014). Diabetes is a recognized independent risk factor for stroke and is associated with higher morbidity and mortality rates (Chen et al., 2016). Clinical epidemiological investigations have documented that diabetic patients are 2 to 6 times more susceptible to ischemic stroke; about 30% of stroke patients are diabetic and more than 90% of them comprise type 2 diabetes mellitus (T2DM) (Beckman et al., 2013). Additionally, other cardiovascular metabolic risk factors including obesity, dyslipidaemia, and hypertension are often comorbid with T2DM, and may in concert contribute to higher stroke risks when compared to patients with similar risk profiles without diabetes (Chen et al., 2016). Clinically T2DM stroke patients have nearly double the mortality rate and worse neurological outcomes compared to non-diabetic stroke patients (Air and Kissela, 2007; Tureyen et al., 2011). Importantly T2DM stroke patients respond less favorably to tPA therapy due to lower recanalization rates (Molina et al., 2001; Molina et al., 2004; Tang et al., 2016), but have higher risks of hemorrhagic transformation (Linfante et al., 2002; Kwon et al., 2004).
DM: an unfavorable comorbidity for tPA thrombolytic stroke therapy
1. DM is a risk factor for lower recanalization rates after tPA thrombolysis
Intravenous thrombolysis (IVT) with recombinant tPA is a proven beneficial treatment for AIS when given within 4.5 hr of symptom onset (Jauch et al., 2013). Although patients with AIS and diabetes can achieve substantial benefit from IVT (Reiter et al., 2014), several large clinical trials examining ischemic stroke patients treated with IVT reported associations between DM and/or post-stroke hyperglycemia and unfavorable neurological outcome, hemorrhagic transformation, and death (Linfante et al., 2002; Kwon et al., 2004). Other clinical studies also showed that DM or post-stroke hyperglycemia was associated with lower recanalization rates in IVT treated stroke patients (Molina et al., 2001; Molina et al., 2004; Tang et al., 2016). These clinical studies suggested that there is an impaired fibrinolytic response in the setting of DM and/or hyperglycemia. Although the detailed mechanisms underlying DM-related complications after stroke remain to be defined, we speculate that the following pathological factors might contribute to the reperfusion resistance or lower recanalization rate after IVT in DM stroke patients.
The first pathological factor that we hypothesize may underlie lower recanalization rates in stroke patients with DM is higher plasma plasminogen activator inhibitor 1 (PAI-1) levels. Hypercoagulative status in DM is mainly attributed to elevated platelet activation and higher circulating PAI-1 levels, which might be partially responsible for the lower recanalization rate of IVT (Pandolfi et al., 2001; Vaidyula et al., 2006; Lemkes et al., 2010; Tjarnlund-Wolf et al., 2012). It has been shown that diabetes and metabolic syndrome are associated with increased plasma PAI-1 levels in patients at risk of atherothrombosis (Alessi et al., 2007; Aso, 2007). Biologically, PAI-1, the main and potent endogenous t-PA inhibitor, could potentially affect the therapeutic efficacy of exogenously administered t-PA by inhibiting its actions. Although clinical investigation is largely lacking, a few experimental and clinical reports suggest that a fibrinolytic profile upon admission is associated with symptomatic hemorrhagic transformation and recanalization resistance in stroke patients treated with t-PA reperfusion therapy (Ribo et al., 2004a; Ribo et al., 2004b; Montaner, 2009; Walter et al., 2010). It has been shown that lower baseline plasma PAI-1 and thrombin-activable fibrinolysis inhibitor (TAFI) levels predict symptomatic hemorrhagic transformation (Ribo et al., 2004b), whereas higher plasma PAI-1 levels upon admission predict t-PA thrombolytic resistance (Ribo et al., 2004a). However, we should be cautious when using PAI-1 levels as a predictive indicator. In addition, other risk factors, such as atherothrombosis and pre-existing vascular comorbidities in most patients with stroke also need to be considered (Whiteley, 2011). Thus the role of circulating PAI-1 levels in the variable reperfusion rate and hemorrhagic transformation risk following t-PA thrombolytic stroke therapy needs to be further elucidated (Tjarnlund-Wolf et al., 2012).
Another possible pathological factor that may be involved in the lower recanalization rate in DM stroke patients treated with IVT is diabetic atherogenic vascular damage. Systemic inflammation and atherogenic pathological processes in the cerebral vascular walls are present in DM, resulting in elevation of vascular inflammation, BBB integrity disruption, impaired vascular fibrinolysis, and eNOS activity (Lenart et al., 2016). Once ischemic stroke occurs under diabetic state, there may be more robust platelet activation, fibrin deposition, circulating inflammatory cell accumulation, and new thrombosis formation at the occluded vascular injury site, which might lead to thrombolytic resistance (Ly et al., 2017; Venkat et al., 2017).
One more potential pathological factor underlying the low recanalization rate with DM is glycation of tPA thrombolytic receptor annexin A2. Annexin A2 is a fibrinolytic receptor of tPA that acts to accelerate tPA-converted plasmin generation (Ling et al., 2004). Our previous studies showed recombinant annexin A2 (rA2) in combination with low-dose tPA improved thrombolytic efficacy and long-term neurological outcomes after embolic focal ischemia in rats (Wang et al., 2014; Jiang et al., 2015; Fan et al., 2017). Impaired fibrinolysis on the surface of endothelial cells has been identified as a key pathological factor in thrombotic vascular complications in patients with diabetes (Alzahrani and Ajjan, 2010). An immunochemical and biochemical study examining endothelial plasma membrane proteins that are glycated in diabetes, has identified annexin A2 as one of the three major glycated proteins labeled by the anti-glucitollysine (Ghitescu et al., 2001). In cultured human brain microvascular endothelial cells, we found hyperglycemia for 7 days significantly reduced cell surface fibrinolytic activity, and also decreased tPA, plasminogen, and annexin A2 mRNA and protein expressions, while increasing PAI-1 levels. Hyperglycemia significantly increased advanced glycation end products (AGE)-modified forms of total cellular and membrane annexin A2. The hyperglycemia-associated reduction in fibrinolytic activity was fully restored upon incubation with recombinant annexin A2 (rA2). However, neither the hyperglycemia induced increases in AGE-modified annexin A2 nor exogenous tPA were reversed, supporting the hypothesis that hyperglycemia causes dysfunction in the endothelial membrane protein annexin A2, thereby leading to an overall reduction in fibrinolytic activity (Dai et al., 2013). Our experimental findings also support the previous speculation that impaired fibrinolysis due to glycation of endothelial annexin A2 may be partially responsible for worse neurological outcome and resistance to tPA reperfusion in diabetic stroke complications, as an acquired annexinopathy (Gugliucci and Ghitescu, 2002).
Additionally, a higher fibrin clot density may also be considered a pathological factor for the low recanalization rate in stroke patients with DM. It has been reported that DM patients exhibit increased maximal fibrin clot strength (Maatman et al., 2018) and increased fiber density (Dunn et al., 2005). Prolonged duration of T2DM is associated with a pro-thrombotic fibrin clot phenotype (Konieczynska et al., 2014). These observations suggest a denser fibrin clot in DM patients might also contribute to the increase in tPA thrombolytic resistance (Dunn et al., 2006; Tang et al., 2016). Lastly, impaired collateral flow compensation may also underlie the low recanalization rate in stroke patients with DM (Kimura et al., 2009; Liebeskind et al., 2014). Cerebral vasoreactivity and collateral circulation are important protective mechanisms against cerebral ischemia. Clinical investigations have suggested that more robust collateral grade was associated with better recanalization, reperfusion, and subsequently better clinical outcomes (Liebeskind et al., 2014). Experimental studies have documented impaired collateral flow compensation during chronic cerebral hypoperfusion and after focal ischemic stroke in mice with T2DM (Akamatsu et al., 2015; Nishijima et al., 2016), suggesting that the impaired collateral flow might not directly affect thrombolysis, but may contribute to the lower reperfusion efficacy of tPA thrombolytic therapy and worse neurological outcomes in DM stroke patients (Kimura et al., 2009; Tang et al., 2016).
2. DM is a risk factor for tPA-induced intracerebral hemorrhagic transformation
Symptomatic intracranial hemorrhage (SICH) is a devastating complication of intravenous thrombolysis treatment that is associated with high mortality (Seet and Rabinstein, 2012). SICH rates, reported to be from 3-7%, vary considerably between studies and these differences may relate to the differences in the criteria used to define SICH (Seet and Rabinstein, 2012; Reiter et al., 2014). There are a number of risk factors associated with tPA stroke therapy-mediated hemorrhagic transformation, including post-stroke hyperglycemia, older age, larger infarct, and high blood pressure (Miller et al., 2011; Faigle et al., 2014). Post-stroke hyperglycemia is present in all preexisting diabetes patients (about 37% of stroke patients), and 50% of non-diabetic stroke patients (Allport et al., 2006; Kruyt et al., 2010). History of DM, combined with the severity of post-stroke hyperglycemia, is associated with poor clinical outcome after stroke and thrombolysis (Ribo et al., 2005; Bruno et al., 2008; Poppe et al., 2009; Ahmed et al., 2010; Desilles et al., 2013). For example, in the NINDS r-tPA Stroke Trial, in patients treated with tPA within 3 hours of onset, serum glucose level was an independent predictor that suggested direct correlation with symptomatic hemorrhagic transformation (Bruno et al., 2002). This was replicated in the PROACT II trial, where symptomatic hemorrhagic transformation occurred in 35% of patients with serum glucose values greater than 200 mg/dL (Kase et al., 2001). In another study using data from the prospective, multicenter Canadian Alteplase for Stroke Effectiveness Study (CASES), in the cohort of IV-tPA–treated stroke patients, admission hyperglycemia was independently associated with increased risk of death, hemorrhagic transformation, and poor functional status at 90 days (Poppe et al., 2009). Although evidence supports an increased risk of hemorrhage due to tPA in DM patients with hyperglycemia, the mechanisms underlying this effect remain to be fully elucidated (Ishrat et al., 2012; Fan et al., 2014; Whiteley et al., 2014; Kanazawa et al., 2017).
Interactions among multiple factors, including hyperglycemia-mediated vascular oxidative stress, neuroinflammation-mediated injury, ischemic insult, and tPA neurovascular toxicity contribute to the extracellular proteolysis dysfunction- BBB damage-intracerebral hemorrhagic transformation process (Lo et al., 2002; Wang and Lo, 2003; Wang et al., 2004; Won et al., 2011; Hafez et al., 2014). Hyperglycemia may cause vascular oxidative stress, which occurs very early after the onset of ischemia/reperfusion injury via overproduction of reactive oxygen species (ROS). Oxidative stress generated during stroke is a critical event leading to BBB disruption with secondary vasogenic edema and hemorrhagic transformation of infarcted brain tissue, restricting the benefit of thrombolytic reperfusion (Kaur et al., 2004; Wang et al., 2004). ROS can also directly oxidize and damage BBB structures. Furthermore, ROS is an upstream intermediate of pathophysiological mechanisms during reperfusion injury that links protease activation to vascular leakage (Gasche et al., 2001; Jian Liu and Rosenberg, 2005). The importance of oxidative stress in stroke and tPA thrombolytic-related vasculature disruption has been well-documented in several animal studies employing antioxidant plus tPA combination treatments in embolic stroke models (Asahi et al., 2000; Lapchak et al., 2001; Lapchak et al., 2002). In human stroke, increased oxidative stress and its direct relationship to matrix metallopeptidase (MMP)-9 expression was observed (Kelly et al., 2008), in accordance with preclinical animal studies.
Post-ischemic neuroinflammation-mediated BBB leakage after stroke is a progressive and interactive process, and largely depends on the activation, expression, and secretion of proinflammatory mediators (e.g. cytokines) from both cerebral and peripheral cells (Rosenberg, 2002; Gidday et al., 2005 ; Amantea et al., 2013). Oxidative stress is a major stimulator of inflammatory cytokine production and protease secretion by microglia, leukocytes, and brain resident cells of the neurovascular unit (Wang and Lo, 2003; Lee et al., 2004; Pun et al., 2009). Experimental investigations have suggested that neuroinflammation-mediated extracellular matrix proteolysis dysfunction is the key pathological mechanism contributing to BBB disruption after stroke, mainly by means of early elevated cytokines, release of leukocytes into circulation, adhesion of leukocytes to the injured cerebrovasculature, leukocyte brain infiltration, and release and activation of proteases (Simi et al., 2007; Najjar et al., 2013). Leukocyte-microvessel interactions, and subsequent infiltration of leukocytes into the ischemic brain play prominent roles in the development of secondary damage (Amantea et al., 2013), resulting in edema, microvascular permeabilization, and hemorrhage via secreted free radicals, cytokines/chemokines, lipid-derived mediators, and proteases (Wang and Lo, 2003; Fagan et al., 2004; Borlongan et al., 2012). It has been clearly recognized that proteases secreted by activated leukocytes is one of key pathological factors contributing to BBB leakage and hemorrhagic transformation in ischemic stroke (Lo et al., 2002; Wang and Lo, 2003; Lee et al., 2004; Bao Dang et al., 2013). Importantly, activated extracellular proteases such as MMP-9 act as inflammatory mediators as well, for example, by triggering cytokine expression (Radisky et al., 2005; Amantea et al., 2007; Bao Dang et al., 2013).
In the context of extracellular proteolytic dysregulation in tPA stroke thrombolysis-related hemorrhagic complication, the primary focus is based on the interaction between tPA and MMP-9 (Adibhatla and Hatcher, 2008; Wang et al., 2008; Jin et al., 2010; Lakhan et al., 2013). tPA amplifies MMP-9 expression, which may increase oxidative stress and lead to neuroinflammation (Lo et al., 2004; Wang et al., 2004), Lakhan et al., 2013, Wang et al., 2003; Zhang et al., 2007). Experimental data suggest that extracellular matrix proteolysis may target multiple cell types at the neurovascular interface, and underlie multiple cascades of BBB disruption after tPA reperfusion treatment (Lee et al., 2004; Lo et al., 2004; Seo et al., 2012).
Fundamentally, the precise pathological molecular mechanisms involved in DM induced complications after stroke remain poorly characterized (Desilles et al., 2013; Hafez et al., 2014), especially because assessing dynamic changes and interactions in multiple factors is technically difficult, particularly in in vivo animal models. As discussed earlier, because there are multiple upstream regulators of neurovascular proteolysis-BBB disruption comprising mediators of hyperglycemia, oxidative stress, and inflammation, exogenous tPA may contribute to this pathological process (Kaur et al., 2004; Lo et al., 2004; Adibhatla and Hatcher, 2008; Wang et al., 2008; Jin et al., 2010; Fan et al., 2014). Therefore, in combination with tPA in patients with diabetes/post stroke hyperglycemia, targeting these upstream mechanisms, or even multiple combinations of these mechanisms may ultimately improve stroke thrombolytic therapy in both safety and efficacy (Fan et al., 2014; Kanazawa et al., 2017; Knecht et al., 2018).
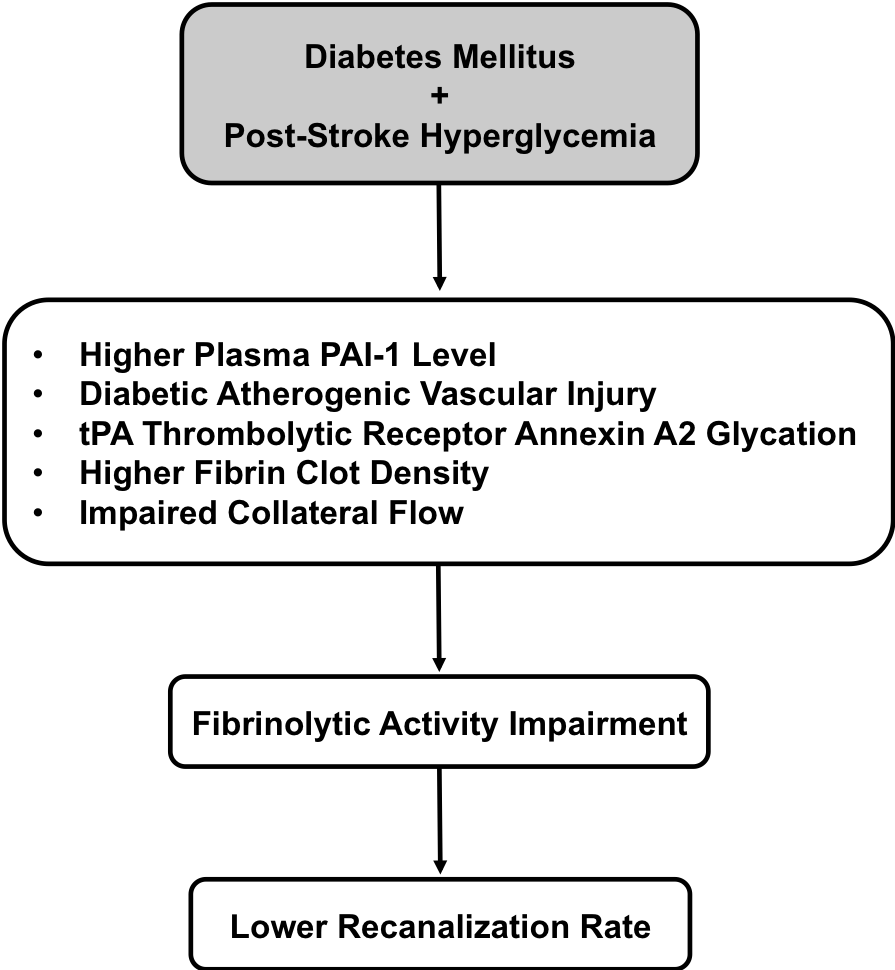
In a new window | Download PPT
Figure 1: A schematic outline to link pathological association between diabetes mellitus plus post stroke hyperglycemia and lower recanalization rate after tPA thrombolytic stroke therapy. The potential pathological factors that underlying this therapeutic shortcoming include higher plasma PAI-1 level, diabetic atherogenic vascular injury, glycation of tPA thrombolytic receptor annexin A2, higher fibrin clot density, and impaired collateral flow. These factors all contribute to fibrinolytic activity impairment in exogenous tPA that clinically presents as a lower recanalization rate.
Summary
DM is recognized as a clinically important vascular co-morbidity that leads to lower recanalization rates and increased risks of hemorrhagic transformation. In this mini-review, we summarized recent advances in the understanding of the underlying mechanisms involved. Potential pathological factors that relate to suboptimal recanalization include higher plasma PAI-1, diabetic atherogenic vascular damage, glycation of the tPA receptor annexin A2, alterations in fibrin clot density, and impaired collaterals (Figure 1). Factors that may contribute to hemorrhagic transformation include hyperglycemia, vascular oxidative stress, inflammation, tPA neurovascular toxicity, and dysfunction in extracellular proteolysis balance (Figure 2). A better understanding of these complex pathways may eventually lead to novel ways of counteracting the negative effects of DM in stroke.

In a new window | Download PPT
Figure 2: A schematic outline to link pathological association between diabetes mellitus plus post stroke hyperglycemia and lower recanalization rate after tPA thrombolytic stroke therapy. The potential pathological factors that underlying this therapeutic shortcoming include higher plasma PAI-1 level, diabetic atherogenic vascular injury, glycation of tPA thrombolytic receptor annexin A2, higher fibrin clot density, and impaired collateral flow. These factors all contribute to fibrinolytic activity impairment in exogenous tPA that clinically presents as a lower recanalization rate.
The conflicts of interest statement
The authors declare that they have no conflicts of interest.
References
Yinghua Jiang
1Department of Neurosurgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Ning Liu
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
3The Third Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Zeyuan Cao
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Lena Huang
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Zhanyang Yu
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Qiuchen Zhao
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
4Department of Neurology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, Jiangsu 210008, China.
Fang Zhang
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
5Department of Neurology, Tianjin Neurological Institute, Tianjin Medical University General Hospital, Tianjin 300052, China.
Ming-Ming Ning
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Klaus van Leyen
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Eng H. Lo
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Xiaoying Wang
2Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, USA.
Corresponding author:
Xiaoying Wang
Email: wangxi@helix.mgh.harvard.edu

In a new window | Download PPT
Figure 1: A schematic outline to link pathological association between diabetes mellitus plus post stroke hyperglycemia and lower recanalization rate after tPA thrombolytic stroke therapy. The potential pathological factors that underlying this therapeutic shortcoming include higher plasma PAI-1 level, diabetic atherogenic vascular injury, glycation of tPA thrombolytic receptor annexin A2, higher fibrin clot density, and impaired collateral flow. These factors all contribute to fibrinolytic activity impairment in exogenous tPA that clinically presents as a lower recanalization rate.

In a new window | Download PPT
Figure 2: A schematic outline to link pathological association between diabetes mellitus plus post stroke hyperglycemia and lower recanalization rate after tPA thrombolytic stroke therapy. The potential pathological factors that underlying this therapeutic shortcoming include higher plasma PAI-1 level, diabetic atherogenic vascular injury, glycation of tPA thrombolytic receptor annexin A2, higher fibrin clot density, and impaired collateral flow. These factors all contribute to fibrinolytic activity impairment in exogenous tPA that clinically presents as a lower recanalization rate.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 14777 | 59 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA