Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Gut microbial metabolites as immunomodulators in acute brain injury
Time:2021-04-05
Number:12939
Diana Fink1, Isha Yogesh1, Alexander Beer1, Rosa Delgado Jimenez1, Corinne Benakis1
Author Affiliations


- 1Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Germany
Conditioning Medicine 2021. 4(1):28-38.
Abstract
Abstract: Stroke and traumatic brain injury are leading causes of death and disability. Although these acute brain disorders affect different age groups, both share similar pathobiological mechanisms and result in immediate as well as long-term motor and cognitive dysfunction. During the past two decades, the gut microbiota has been revealed to play a key role in the pathophysiology of cerebral ischemia and brain trauma. In particular, the interaction between the gut microbiota and the host immune system was shown to greatly influence recovery in animal models of stroke. Yet, the functional profile of a specific microbiota influencing the immune response to brain injury remains to be defined. Knowing that microbial metabolites are the molecular basis for the host immune system-microbiota crosstalk, specific gut metabolic pathways could be targeted to mediate recovery after brain injury. In this short review, we summarize the recent literature on the possible role of gut metabolites with immunomodulatory properties that could influence the neuroinflammation in acute brain injuries and promote recovery.
Keywords: Stroke, Traumatic brain injury, Microbiota, Neuroinflammation, Short chain fatty acids, Metabolites
Abstract
Abstract: Stroke and traumatic brain injury are leading causes of death and disability. Although these acute brain disorders affect different age groups, both share similar pathobiological mechanisms and result in immediate as well as long-term motor and cognitive dysfunction. During the past two decades, the gut microbiota has been revealed to play a key role in the pathophysiology of cerebral ischemia and brain trauma. In particular, the interaction between the gut microbiota and the host immune system was shown to greatly influence recovery in animal models of stroke. Yet, the functional profile of a specific microbiota influencing the immune response to brain injury remains to be defined. Knowing that microbial metabolites are the molecular basis for the host immune system-microbiota crosstalk, specific gut metabolic pathways could be targeted to mediate recovery after brain injury. In this short review, we summarize the recent literature on the possible role of gut metabolites with immunomodulatory properties that could influence the neuroinflammation in acute brain injuries and promote recovery.
Keywords: Stroke, Traumatic brain injury, Microbiota, Neuroinflammation, Short chain fatty acids, Metabolites
Introduction
Stroke and traumatic brain injury (TBI) are both acute brain disorders that trigger a strong inflammatory response starting locally in the brain and propagating through peripheral organs (Shi et al., 2019; Bramlett and Dietrich, 2004). It is now well established that the innate and adaptive immune system plays a pivotal role in the evolution of cerebral injury, by actively participating in brain damage development on the one hand, and in tissue repair on the other (Iadecola and Anrather, 2011; Russo and McGavern, 2016). Attempts to modulate the immune response in stroke patients and after head injury mitigated deleterious outcomes (Zhu et al., 2015), or led to either no effects or negative effects in clinical trials (Edwards et al., 2005; Wright et al., 2014; Elkind et al., 2020). These clinical studies warranted the need to further investigate how the immune response to brain injury is regulated. The gastrointestinal tract (GI) contains the largest pool of immune cells in the body (Mowat and Agace, 2014), and immune homeostasis is tightly regulated by trillions of microorganisms (Ivanov and Honda, 2012), collectively named the gut microbiota. Through constant contact with commensal bacteria and microbial-derived molecules, epithelial and immune cells in the gut have developed the capacity to maintain a homeostatic state by defending host integrity while promoting tolerogenic responses (Ivanov and Littman, 2011). During the past two decades, the crosstalk between the microbiota and the intestinal immune system has been demonstrated to greatly influence brain health and diseases (Cryan et al., 2019), including stroke (Delgado Jiménez and Benakis, 2020) and TBI (Zhu et al., 2018). Indeed, recent findings in rodents and patients demonstrated that stroke and brain trauma altered the composition of the gut microbiota – known as dysbiosis (Yamashiro et al., 2017; Urban et al., 2020). This effect was associated with a stress response in the gut, intestinal barrier dysfunction, and bacterial translocation, which together participate in the imbalance of the inflammatory response in the gut, an increase in intestinal immune cell trafficking to the brain, and ultimately to the exacerbation of the neuroinflammatory response to brain injury in a vicious cycle (Figure 1) (Benakis et al., 2016; Houlden et al., 2016; Singh et al., 2016; Stanley et al., 2016; Treangen et al., 2018; Benakis et al., 2020). These experimental findings identify the gut-brain immune axis as a novel component of the inflammatory response to acute brain injury. However, to date, it is still not known which types of gut bacteria participate in the intestinal immune changes after stroke. In addition, the microbial cues influencing the immune cells in the gut after stroke remain to be identified. Indeed, the causal mechanisms underpinning gut microbiota and host immune system crosstalk in acute brain injuries remain largely unknown. The vast microbial ecosystem performs essential metabolic functions including vitamin biosynthesis, carbohydrate fermentation, bile acid metabolism, and importantly shapes the host immune system. Immunomodulatory function of commensal bacteria is mediated by thousands of microbial-derived molecules and metabolites with local and systemic effects (Schroeder and Bäckhed, 2016). These microbial molecules can interact with the mucosal epithelial and intestinal immune cells, stimulate the vagus nerve, or reach the systemic circulation to signal to the brain (Needham et al., 2020) and possibly, through one or more of these three modes of actions, modulate the neuroinflammatory response to acute brain injury. Thus, bacterial products are promising candidates in microbiota-mediated immunomodulation in stroke and TBI. The most known metabolite classes derived from the gut microbiota with immunomodulatory effects are: 1) amino acids and their derivatives; 2) products of microbial fermentation: short-chain fatty acids (SCFA) and polyphenols; 3) secondary bile acids; and 4) trimethylamines (Figure 2).
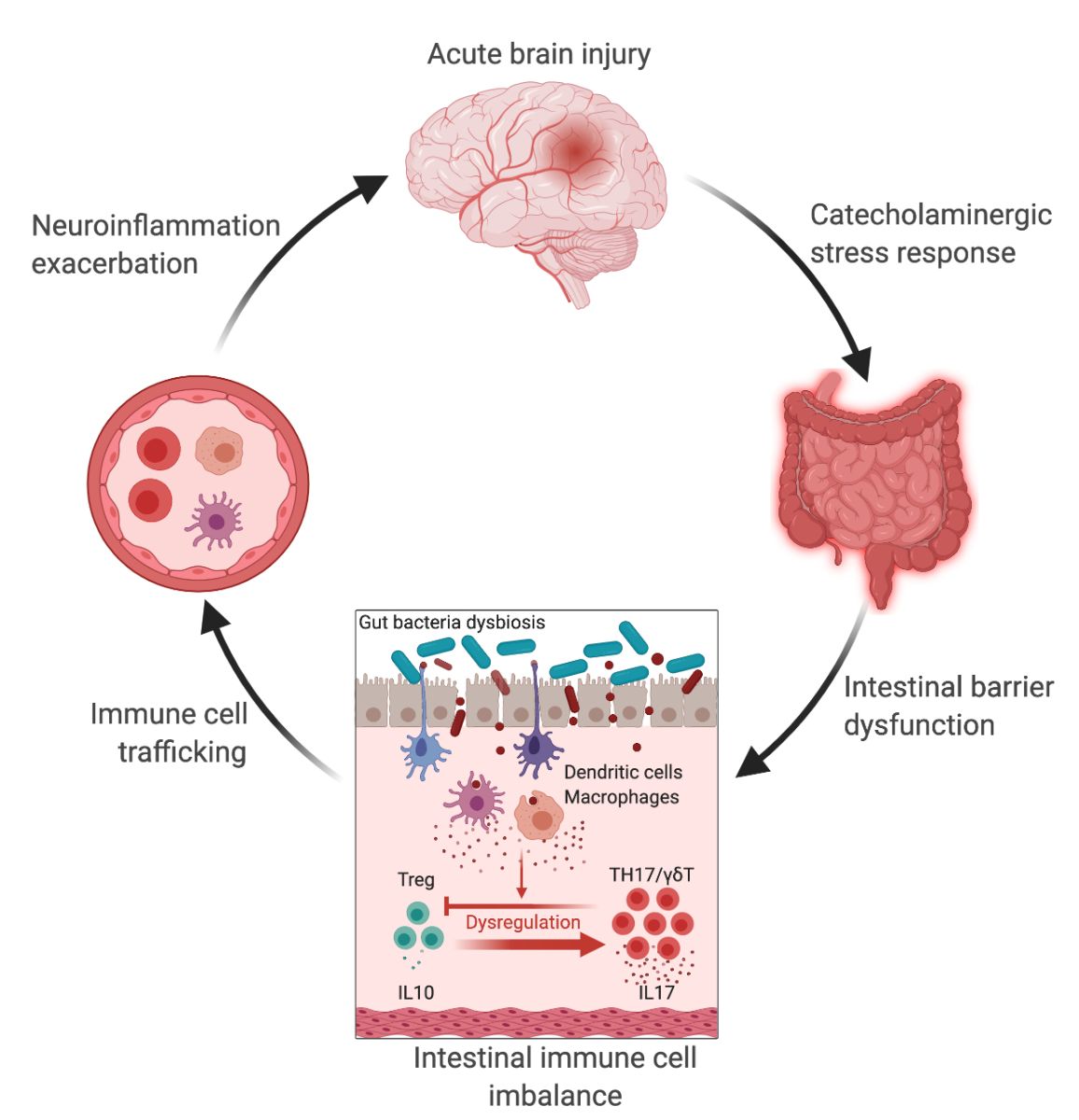
In a new window | Download PPT
Figure 1: The gut-brain axis in acute brain injury. Acute brain injury induces microbiota dysbiosis and is associated with a stress response, intestinal barrier dysfunction, and immune imbalance. These phenomena are relayed to the brain via intestinal immune cell trafficking, increasing the neuroinflammatory response in a vicious cycle (Singh et al., 2016; Houlden et al., 2016; Stanley et al., 2016). Artificial change of the gut microbiota composition using antibiotics, reduces the development of the brain lesion in a mouse model of cerebral ischemia, improves sensory-motor functions caused by microbiota-dependent priming of dendritic cells inducing an expansion of regulatory T cells (Treg) suppressing γδT-IL17+ cells (Benakis et al., 2016). These findings suggest that the gut microbiota can influence development of the brain lesion via modulation of the microbiota–intestinal immune system interaction. Created with BioRender.com.
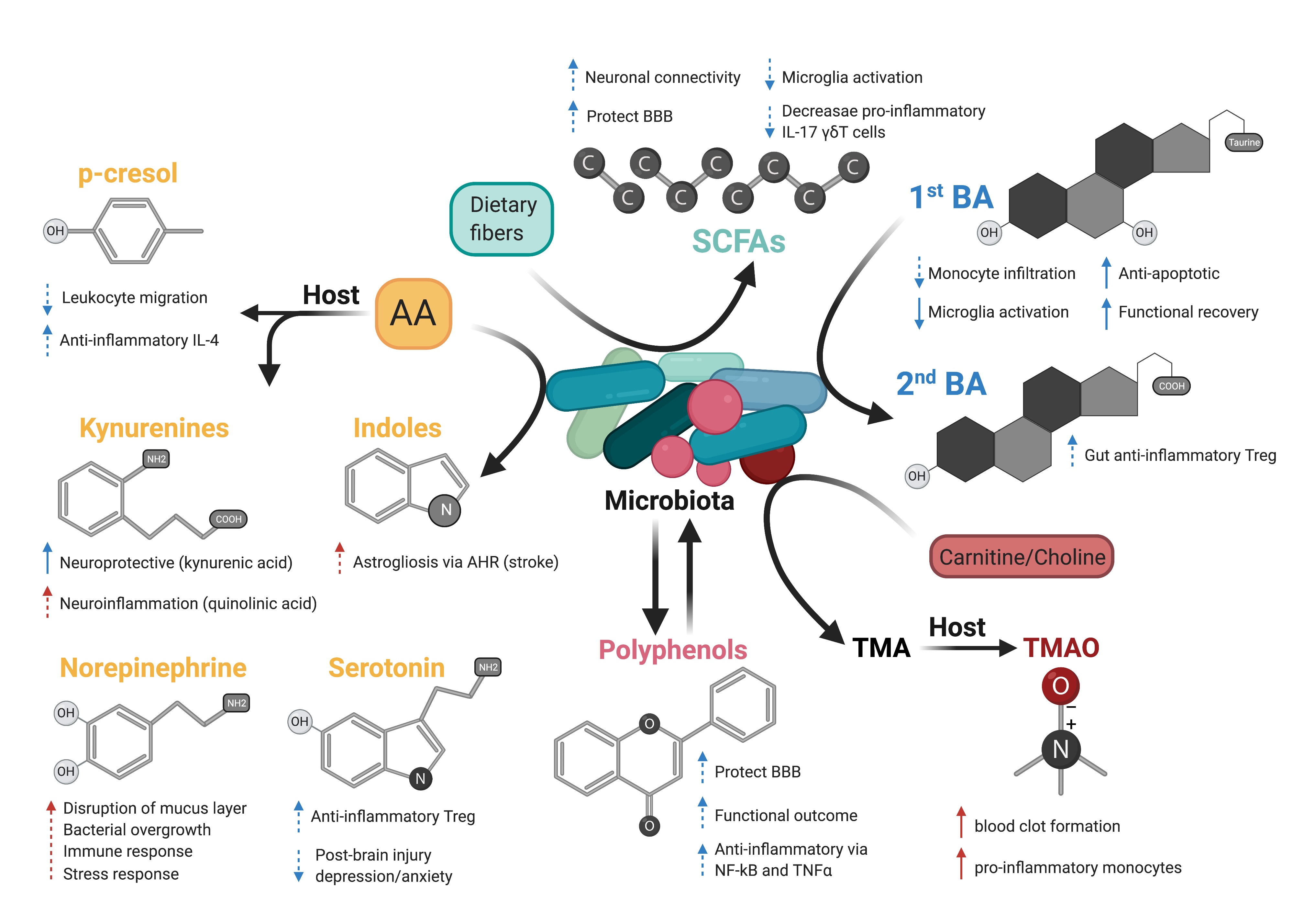
In a new window | Download PPT
Figure 2: Possible effects of microbiota-derived metabolites on acute brain injury. Dash arrows indicate that a direct link has not been described. Blue and red arrows indicate a favorable outcome or deleterious outcome, respectively. Created with BioRender.com.
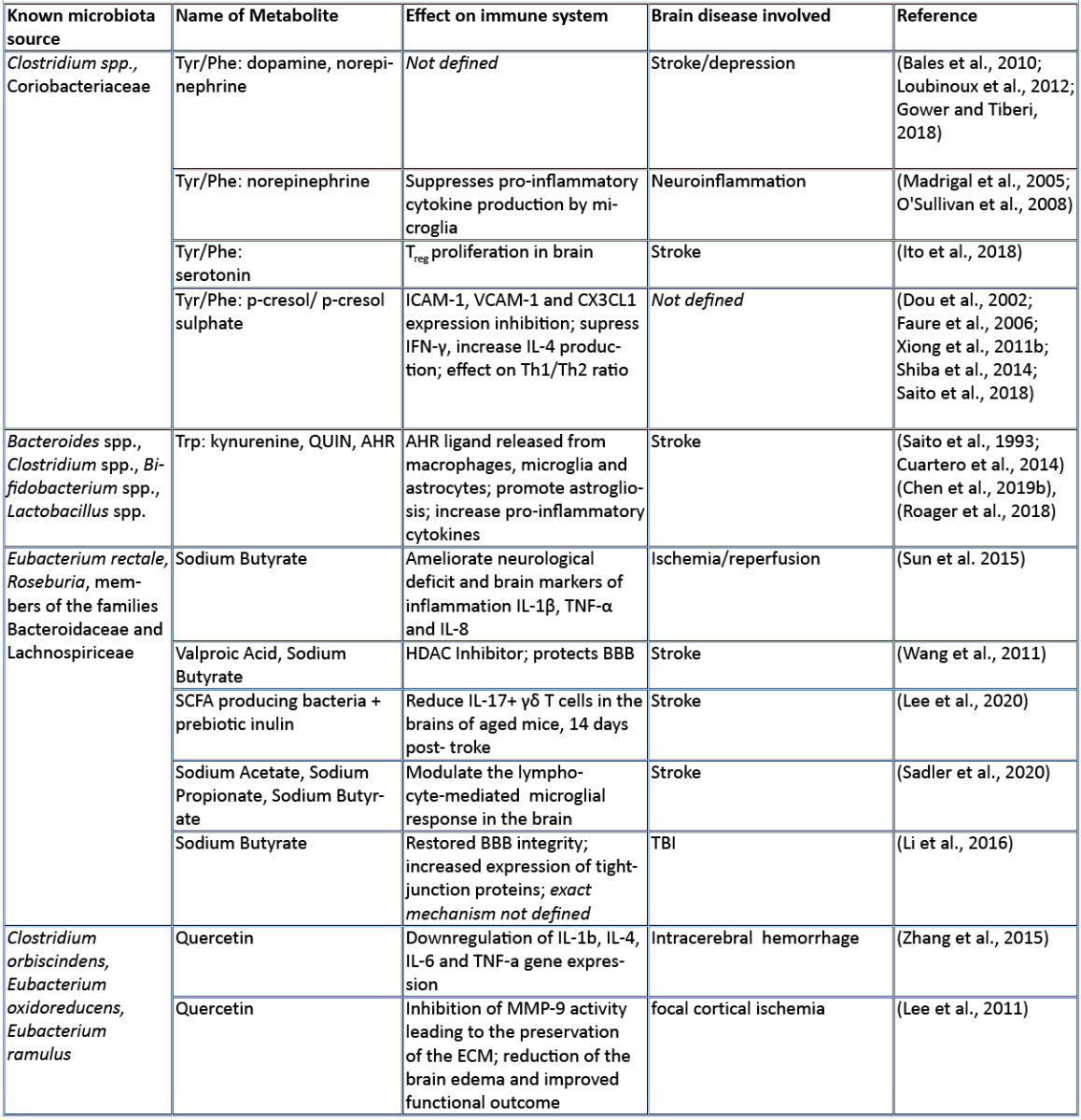
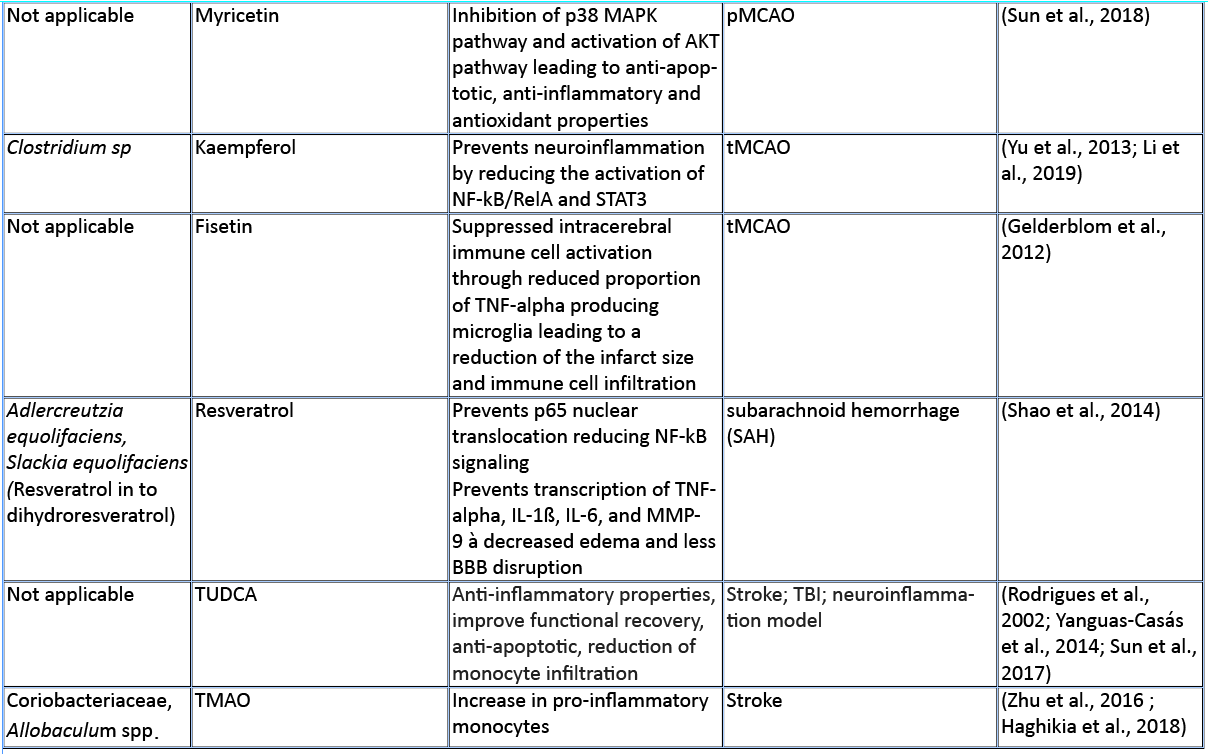
In this review, we aim to summarize the current knowledge on how these microbiota-derived metabolites influence the host immune system focusing on stroke and TBI (Table 1). The role of microbial metabolites in acute brain injuries is still scarce, especially concerning TBI and intracerebral hemorrhage, thus this current review mainly summarizes findings in cerebral ischemia. Nevertheless, we hope to give here new possible research paradigms focusing on metabolic pathways of the gut microbiota–host immune system crosstalk in acute brain injuries. Reshaping the microbiota-brain interaction by bioactive microbial metabolites could bring new therapeutic avenues to acute brain injuries and promote recovery.
Amino acids
Amino acids (AA) and their bioproducts of commensal bacteria metabolism not only act as nutrients to the host, being key components of proteins and energy source, but also serve as important regulators of the host immunity (Wu, 2009). The essential aromatic AAs tryptophan and phenylalanine are sourced from the diet in dairy products, meat, and soybeans (Richard et al., 2009; Rocha and MacDonald, 2016), making them interesting targets for diet intervention in high-risk stroke patients. The aromatic AAs tryptophan, phenylalanine, and phenylalanine-derived tyrosine are metabolized into a large number of downstream metabolites including dopamine, norepinephrine, and serotonin (Dodd et al., 2017; Mittal et al., 2017), as well as the immunomodulators p-cresol and indoles. Whereas synthesis of catecholamines is mainly regulated by the host, their availability also depends on phenylalanine/tyrosine precursors and the enzymatic activity of commensals (Kessel et al., 2019). Interestingly, Houlden et al. (2016) demonstrated a local increase in the stress hormone norepinephrine in the gut after brain injury, which correlated with changes in the microbiota composition and a disruption of the mucus layer. These microbially synthesized neurotransmitters can promote pathogens and bacterial growth (Lyte, 2013). Opportunistic bacteria and/or norepinephrine could possibly penetrate the damaged intestinal layer, and trigger a local immunomodulatory function. Alternatively, small neuroactive molecules can be systemically dispersed by the blood stream and enter the brain where they can have a local effect. Interestingly, serotonin promotes proliferation of regulatory T cells (Treg) cells in the brain and improves neurological score, evaluated by the a four-point scale neuroscore (Funato et al. 1997) and the corner-turn test (Balkaya et al., 2013), 14 days after the onset of stroke in mice (Ito et al., 2018). Since the release of norepinephrine, serotonin, and dopamine in the brain has been involved in the modulation of anxiety and depression behavior following brain injury (Madrigal et al., 2005; O'Sullivan et al., 2008), it would be of interest to investigate whether the microbiota can modulate the catecholaminergic system in the gut and further influence the stress response in the brain. Interestingly, Yano et al. (2015) showed that gut microbial populations dominated by Clostridium species increase serotonin production by promoting its biosynthesis in colonic enterochromaffin cells, the connection with the brain is however yet to be described. On the other hand, germ-free mice (GF) had increased levels of stress hormones, which were correlated with decreased expression of brain-derived neurotrophic factor (Sudo et al., 2004). However, to date, there is no evidence regarding the direct effect of microbiota-induced neurotransmitters in stroke and TBI. Nevertheless, modulation of phenylalanine/tyrosine metabolism by the gut microbiota could open novel opportunities to target microbial-derived neuroactive compounds in brain injury, especially targeting long-term comorbidities such as depression and stress behaviour (Kapoor et al., 2017; Milders et al., 2003).
Predictive analysis of the gut microbial enzymatic signature after cerebral ischemia in rodents revealed that aromatic compound degradation discriminates highly between large versus small infarct size (Benakis et al., 2020). Interestingly, several pathways related to aromatic compound metabolism (benzoate degradation, phenylalanine, tryptophan, and histidine metabolism) were associated with the microbiota composition of ampicillin-treated mice-inducing neuroprotection in experimental stroke (Benakis et al., 2020). This suggest that microorganisms involved in aromatic-AA metabolism might be involved in the development of the ischemic infarct (Stanley et al., 2018). Several reports identified a shift in the microbiota composition after stroke and TBI that could be involved in the metabolism of AA (Singh et al., 2016; Spychala et al., 2018; Treangen et al., 2018; Angoa-Pérez et al., 2020). Likewise, the aromatic AA phenylalanine/tyrosine can be metabolized by various bacterial species found in the intestinal microbiota – including Clostridium species of the Firmicutes phyla – into several immunomodulators such as p-cresol metabolite (Saito et al., 2018). Whereas the direct implication of Clostridium bacteria in the metabolism of p-cresol has not been investigated yet in brain injury, p-cresol has several immunomodulatory properties. Indeed, it inhibits cytokine induced expression of the intracellular adhesion molecule 1 (ICAM-1) and the vascular cell adhesion molecule (VCAM-1) on endothelial cells (Dou et al., 2002) – important for leukocyte transendothelial migration (Faure et al., 2006) – as well as the increase in interleukin (IL)-4 production from Th2 cells in vitro (Shiba et al., 2014). These immunomodulatory effects of p-cresol are of relevance for stroke since cell adhesion molecule blockage diminishes the secondary inflammatory response after ischemic stroke (Liesz et al., 2011a; 2011b). In addition, IL-4 knock-out mice that undergo transient middle cerebral artery occlusion (MCAO), show an increase in Th1/Th2 ratio, increase in microglia/macrophage numbers, and suffer from greater ischemic damage (Xiong et al., 2011a). With p-cresols’ known effect on IL-4 production and leukocyte transmigration, it could serve as a possible immune-regulator in brain injury.
Tryptophan and its derived-metabolites have been shown to play a crucial role in regulating inflammation and disease development both in the gut and the central nervous system (Lamas et al., 2018; Rothhammer et al., 2016). Specifically, the increase in tryptophan catabolism is associated positively with the severity of stroke outcome in patients (Brouns et al., 2010; Ormstad et al., 2013). Tryptophan can be metabolized through three different pathways: the kynurenine pathway mainly by immune and epithelial cells, the serotonin pathway by enterochromaffin cells, and the indole pathway by the gut microbiota (Agus et al., 2018).
The kynurenine pathway accounts for more than 90% of the tryptophan metabolism and has been involved in several neurodegenerative diseases (Schwarcz et al., 2012) and acute brain disorders. Likewise, the kynurenine-derived metabolite quinolinic acid is increased in the brain two days after ischemic stroke and persists for up to seven days post ischemia (Saito et al., 1993). While quinolinic acid is deleterious to the brain after stroke, kynurenic acid has a protective role as an antagonist of N-methyl-D-aspartate receptors (NMDA) (Cuartero et al., 2016).
Furthermore, indole breakdown products of tryptophan occur by various bacterial species such as Bacteroides spp., Clostridium spp., Bifidobacterium spp., and Lactobacillus spp. (Roager and Licht, 2018), and serve as critical immunomodulators (Quintana et al., 2008). Several indole metabolites bind to and activate the aryl hydrocarbon receptor (AHR) – a transcription factor involved in the immune response (Quintana, 2013; Rothhammer and Quintana, 2019). AHR ligands comprise, not exclusively, indole-3-propionic acid (IPA), indole-3-acetic acid (IAA), indole acrylic acid (IA), and indoxyl-3-sulfate (I3S, partially metabolized in the liver) (Agus et al., 2018; Vyhlídalová et al., 2020). They are absorbed through the intestinal wall and interact with intestinal immune cells or enter the blood stream where they can have a systemic effect on remote organs such as the brain (Roager and Licht, 2018). AHR is expressed in several immune cells of the innate and adaptive immune system including pro-inflammatory TH17, anti-inflammatory regulatory T cells (Treg), and dendritic cells (DCs), as well as in brain resident glial cells (Rothhammer and Quintana, 2019). In experimental autoimmune encephalomyelitis (EAE) the lack of tryptophan metabolites worsens EAE. Both I3S and IPA limit EAE development. I3S crosses the blood brain barrier (BBB), activates AHR in astrocytes and limits nuclear factor-κB (NF-κB) pro-inflammatory activity in microglia (Rothhammer et al., 2016; 2018). In contrary, AHR is regulated after stroke leading to an increase in astrogliosis and neurogenesis suppression. Likewise, AHR knock-out mice have a better stroke outcome associated with a downregulation of pro-inflammatory cytokines and an increase in neuronal differentiation factors (Chen et al., 2019b). Microbiota-derived tryptophan catabolites are therefore interesting targets to modulate the immune and neuronal response to brain injury.
SCFA
SCFAs are a class of metabolites primarily released during anaerobic fermentation of dietary fibers in the distal gut. They are also produced by amino acid fermentation, but by less than 1% of colonic bacteria. The three main SCFAs are acetate, propionate, and butyrate, and they occur in an estimated ratio of 60:20:20, respectively (Cummings et al., 1987; Silva et al., 2020). Most anaerobic gut bacteria produce acetate as a product of fermentation. The production of butyrate involves carbohydrate glycolysis and members of the colonic Firmicutes phylum, including the Ruminococcaceae and Lachnospiraceae families, as well as the Ersypelotrichaceae and Clostridaceae families. Propionate formation occurs via the succinate or propanediol pathway, by the action of various members of the Bacteriodetes and Firmicutes phyla (Louis and Flint, 2017). For more details about the metabolic pathways of SCFA production, and the microbial species involved, we refer the reader to the review by Louis and Flint (2017). SCFAs have been shown to have significant effects on the host immune system, the brain, and other organs. SCFAs have an immunomodulatory function inducing T cell differentiation into effector or regulatory cells, depending on the immune context (Park et al., 2015). SCFAs have been directly implicated in brain function, for example mono-colonization by a butyrate-producing bacterium restores the germ-free (GF) BBB deficient integrity and has a crucial role in the maturation of microglia (Erny et al., 2015). In a recent study, SCFA supplementation in mice prior to stroke improved behavioral recovery, modified cortical network connectivity, and changed histological markers of synaptic plasticity, which was associated with improved long-term stroke outcomes (Sadler et al., 2020). These effects were correlated with modification of microglial morphology towards a homeostatic-like state. Despite the fact that volume of the infarct was not affected by SCFA supplementation, this study showed for the first time that the gut metabolite of SCFAs participate in post-stroke recovery (Sadler et al., 2020). Lee and colleagues (2020) recently showed that aged mice tend to have a decreased concentration of SCFAs, and this at least partially contributes to their worse stroke outcomes. When the authors administered prebiotic inulin and select SCFA-producing bacteria to aged mice after stroke, they observed a significant reduction in neurological deficits and depressive-like behavior after stroke when compared to controls treated with inulin or SCFA-producers alone. They also found a significant decrease in IL-17+ γδT cells in the brain of aged mice 14 days after stroke, when compared to vehicle-treated controls. This might suggest an effect of SCFAs in reducing post-stroke inflammation induced by the inflammatory IL-17+ γδT cells (Lee et al., 2020). In both of these studies, SCFAs seemed to reduce the abundance of pro-inflammatory T cells in the brain after stroke, but whether SCFAs directly influence T cell polarization and migration has not been addressed yet.
SCFAs like sodium butyrate (SB) and valproic acid (VPA) have epigenetic properties as histone deacetylase inhibitors (HDACs). In accordance, SB has been shown to modulate microglia in the acute phase of stroke through HDACs (Patnala et al., 2017). Wang and colleagues showed that treatment with VPA and SB reduced the elevation of matrix metalloproteinases-9 (MMP-9), the degradation of tight junction proteins, and the nuclear translocation of NF-κB, which are all induced by MCAO. They showed that the HDAC inhibitors VPA and SB inhibited NF-κB activation and proposed that this could prevent MMP-9 transcription (Wang et al., 2011). In addition, SB administered intra-gastrically 3h after transient bilateral occlusion of the common carotid artery was also shown to ameliorate neurological deficits and brain markers of inflammation, including IL-1β, tumor necrosis factor-α (TNF-α), and IL-8 (Sun et al. 2015). In a mouse model of TBI, intra-peritoneal treatment with SB attenuates neurological deficits, brain edema, neuronal change, and BBB damage, as well as increase in tight junction expression in the brain leading to protection against TBI (Li et al., 2016). Smith et al. (2013) showed that treating GF mice with SCFAs specifically increased the number and frequency of colonic Tregs (cTregs). cTregs limit the proliferation of CD4+ T cells, and the authors demonstrated that adding SCFA to co-cultures of cTregs and effector T cells enhanced the cTreg suppressive capacity (Smith et al., 2013). Together, their study showed the direct role of SCFAs in regulating tolerogenic T cell function and highlighted the potential of SCFAs to manipulate the gut-immune axis in brain injury.
Polyphenols
Polyphenols and their secondary metabolites are also derived from the fermentation of dietary substrates. They are a large class of compounds derived from plant-based foods (fruits and vegetables) and beverages including berry fruits, tea, coffee, and red wine. They are chemically defined by at least one aromatic ring with one or more hydroxyl groups attached (Tsao, 2010). Polyphenols have been associated with reduced risk of cardiovascular disease and health benefits (Parrella et al., 2020).
In general, bioavailability of polyphenols is poor, and just 1-10% of the total intake is detectable in blood and urine samples (Tresserra-Rimbau et al., 2018). Even if it is not finally clarified how polyphenols exert their beneficial properties in light of their low bioavailability, it could be explained by the fact that a plethora of polyphenol metabolites show biological activities (Anhê et al., 2019). Recent studies pointed out that there is a bidirectional relationship between the intestinal microbiota and polyphenols (Fraga et al., 2019; Wiciński et al., 2020). Microorganisms in the large intestine play a fundamental role in determining the bioavailability and activity of polyphenols by transforming them into readily absorbable molecules and bioactive metabolites (Dey, 2019). Quercetin, a polyphenol found widely distributed in food plants, is known to be metabolized by various bacterial strains (Winter et al., 1991; Braune et al., 2001). Moreover, quercetin is capable of modulating the immune system by downregulating the gene expression of proinflammatory cytokines like IL-1ß, IL-4, IL-6, and TNF-alpha in intracerebral hemorrhage (Zhang et al., 2015). Furthermore, it has been shown that quercetin preserves the extracellular matrix after focal cerebral ischemia by reducing the increased level of MMP-9 (Lee et al., 2011). Kaempferol, a flavonol-type polyphenol, usually found in spinach and kale (Dabeek and Marra, 2019), can be transformed into metabolites by Clostridium sp. strains. In an animal model of transient MCAO, kaempferol and its metabolites are able to prevent neuroinflammation by reducing the NF-κB/RelA and signal transducer and activator of transcription 3 (STAT3) pathway in rats (Yu et al., 2013; Li et al., 2019). Resveratrol, a stilbene-type polyphenol present in grapes and red wine is known to be metabolized in to dihydroresveratrol by the gut bacterial strains Adlercreutzia equolifaciens and Slackia equolifaciens (Bode, et al., 2013). It has been demonstrated that resveratrol treatment following subarachnoid hemorrhage (SAH) prevents p65 nuclear translocation reducing NF-kB signaling, and preventing transcription of TNF-α, IL-1ß, IL-6, and MMP-9 leading to decreased edema and less BBB disruption (Shao et al., 2014). Fisetin, a flavonol derived polyphenol, commonly found in plant-foods such as strawberries, apples, onions, and cucumbers (Arai et al., 2000), has been shown to suppress intracerebral immune cell activation by reducing the proportion of TNF-α producing brain resident microglia cells, accompanied by decreased immune cell infiltration into the brain and a reduced infarct volume in transient MCAO mice (Gelderblom et al., 2012). Oral gavage of myricetin – a flavonol-type polyphenol, mostly present in tea, berries, fruits, vegetables, and medicinal herbs – has anti-inflammatory properties through the inhibition of cytokine production, including IL-1ß, TNF-alpha, and IL-6 in an animal model of permanent MCAO (Sun et al., 2018). The therapeutic effect of myricetin in reducing ischemic cerebral injuries might be related to the inhibition of the apoptotic inflammatory p38 mitogen-activated protein kinase (MAPK) pathway, a key regulator of pro-inflammatory cytokines synthesis, and the activation of the AKT pathway (Sun et al., 2018). Dietary polyphenols exert their effects through anti-inflammatory, antioxidant, and antimicrobial properties, and have been associated with beneficial effects in the setting of stroke as depicted in a comprehensive review (Parrella et al., 2020). However, most of these studies have not addressed the direct implication of the gut microbiota on the availability of polyphenols after stroke and TBI.
Bile acids
Bile acids are amphipathic molecules synthetized from cholesterol in the liver and secreted into the intestinal tract to facilitate the digestion and absorption of dietary lipids. Before entering the intestinal lumen, bile acids are conjugated with taurine or glycine amino acids, which increase their hydrosolubility and promotes their excretion in the duodenum. Once in the small intestine the two primary bile acids cholic acid and chenodeoxycholic acid encounter the intestinal flora that further metabolizes them to produce secondary bile acids. The main bacterial transformations encompass deamidation by bile salt hydrolases of conjugated amino acids and dihydroxylation catalyzed by 7α-dehydroxylase enzyme (Ridlon et al., 2006) Other metabolic transformations have been described that give rise to a variety of steroid molecules, enriching the bile acid pool and their properties in the intestine (Wahlström et al., 2016).
Both primary and secondary bile acids have roles beyond their lipid detergent properties, acting as immunomodulators (Chen et al., 2019a). The bile acids-receptors farnesoid X nuclear receptor (FXR) and G protein-coupled bile acid receptor 1 (GPBAR1 or Takeda G-protein receptor 5) are expressed by different immune cells regulating the balance between immunity and tolerance. Whereas primary bile acids are the most potent ligands for FXR, secondary bile acids bind preferentially to GPBAR1 (Fiorucci et al., 2018). Major evidence supporting the role of bile acids in tuning the immune response comes from studies using bile acid knock-out mice. Genetic ablation of FXR and GPBAR1 exacerbates intestinal damage in a murine colitis model when challenged with dextran sodium sulfate and trinitrobenzene sulfonate, along with enhanced leukocyte trafficking in the intestine (Cipriani et al., 2011) (Vavassori et al., 2009). FXR-/- macrophages exhibited a pro-inflammatory phenotype at basal state, characterized by increase secretion of pro-inflammatory cytokines. Accordingly, activation of the FXR receptor by synthetic and natural ligands, protected against disease development in a murine model of colitis (Vavassori et al., 2009). Similar to FXR, activation of GPBAR1 by the synthetic ligand BAR501 elicits an anti-inflammatory response in macrophages and protects against chemically-induced colitis. Ablation of the receptor shifts macrophages polarization towards a pro-inflammatory phenotype and worsens intestinal inflammation (Cipriani et al., 2011).
A recent study has unraveled a role for the secondary bile acid isodeoxycholic acid (isoDCA), generated by Clostridium species, in maintaining the tolerogenic intestinal state. IsoDCA antagonizes FXR activity in dendritic cells (DC), dampening their activation and inducing immunosuppressive forkhead box P3 (FoxP3)+ RAR-related orphan receptor (ROR)γt+ regulatory T cell production in the colon (Campbell et al., 2020).
Besides their peripheral immunomodulatory properties, bile acids are also capable of reaching the central nervous system and signals on neuronal cells (Mertens et al., 2017). In a murine model of acute stoke, intravenous administration of the synthetic tauroursodeoxycholic acid (TUDCA) one hour post-occlusion is neuroprotective, improves neurologic functions, and reduces infarct size (Rodrigues et al., 2002). TUDCA acts by inhibiting neuronal apoptosis by stabilizing the mitochondria membrane and preventing the release of apoptosis-inducing factor from the mitochondria. Injection of TUDCA after TBI also resulted in improved functional recovery. The mechanistic link was attributed to the steroid’s anti-apoptotic activity, i.e. abolishing endoplasmic reticulum stress in a pAkt-related manner and reducing neuronal death (Sun et al., 2017). TUDCA also has anti-inflammatory properties that are implicated in its beneficial effect against brain injury. Treatment with TUDCA diminished glial activation and migratory capacities and reduced invasion of monocytes into the CNS in a model of neuroinflammation (Yanguas-Casás et al., 2014). Whereas the direct implication of the microbiota on the availability of TUDCA after brain injury has not been investigated in this study, it would be of interest to test whether stroke or TBI-inducing dysbiosis affect the level of primary and/or secondary bile acids.
In summary, bile acid modulation through the gut microbiota represent a very attractive therapeutic option for the treatment of brain injury since they exert anti-inflammatory activity, lessen neuronal cell death, as well as inhibit monocyte trafficking and induce tolerogenic T cells in the gut.
Choline and carnitine metabolites
Meat and dairy are rich sources of trimethylamine (TMA), which is metabolized from choline or carnitine by TMA lyase, an enzyme found in the gut microbiota. TMA is further digested in the liver to trimethylamine N-oxide (TMAO). High presence of TMAO in blood increases the platelet clot formation in vivo and has been extensively studied in the context of atherosclerosis (Wang et al., 2015; Zhu et al., 2016; Chen et al., 2017). Interestingly, a choline rich diet alters the microbiome composition with several groups of Clostridia family bacteria being reduced (Oscilospira spp., Lachnospiraae spp., Ruminococcus spp.), while others are increased (Coriobacteriaceae, Allobaculum spp.). This change in microbiome composition increases the activity of microbial TMA lyase, resulting in higher blood clot formation (Zhu et al., 2016). TMAO can thus serve as a risk factor and prognostic marker of stroke (Nam, 2019). Additionally, its presence in the blood of stroke patients was shown to be closely related to levels of proinflammatory monocytes (Haghikia et al., 2018). Diet low in choline and carnitine could thus serve as a prevention tactic for cardiovascular events.
Conclusion and perspectives
In this short review, we highlighted the main microbiota-derived metabolites having immunomodulatory properties and influencing the development of the brain lesion. Metabolites derived from amino acids including kynurenine, SCFAs, polyphenols, and bile acids have been characterized as having direct anti-inflammatory properties on brain inflammation, in particular in modulating microglia function, as well as affecting monocyte migration and infiltration. In contrast, indoles and TMAO seem to enhance inflammation via the activation of monocytes/macrophages. It is less known whether these microbial metabolites modulate lymphocytes in the context of acute brain injury, i.e. inducing the neuroprotective Treg. Since T cells play a role in the development of the injury at later time points, they are an attractive therapeutic target due to a wider window of intervention. Thus, gut metabolites could be targeted to modulate different waves of the neuroinflammatory response, from the acute to the chronic phase of brain injury.
Despite these findings, there is still an important lack of knowledge regarding the functional potential of the gut microbiota in acute brain injury. Experimental research has so far identified that the microbiota composition is altered after stroke and TBI, but the identification of bacteria mediating anti-inflammatory or pro-inflammatory properties is poorly investigated and largely unknown. Considering the high inter-individual variations in humans (Costello et al., 2009), the differences observed between mouse strains, and origins (Sadler et al. 2017) of the microbiota composition, identifying the functional microbial signature responsible for immunomodulatory properties could be a better approach rather than identifying specific bacterial species. Indeed, these small microbial molecules have the potential to bring new treatment avenues to modulate the complex inflammatory response occurring in acute brain injuries. Indeed, microbial metabolites are interesting candidates for the development of microbiota-based therapy, since these small molecules are more conserved between species making translational findings more meaningful. Moreover, metabolites-derived from bacteria can undergo rigorous safety testing, administered at defined and effective doses, and via a less invasive delivery route such as by dietary interventions. It is thus possible to take advantage of the microbiota microenvironment to modulate the host immune system and promote brain protection and recovery.
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
Acknowledgements
This study was supported by the European Commission H2020-MSCA-IF 2016 753893 MetaBiota (CB).
References
Diana Fink1
1Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Germany
Isha Yogesh1
1Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Germany
Alexander Beer1
1Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Germany
Rosa Delgado Jimenez1
1Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Germany
Corinne Benakis1
1Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Germany
Corresponding author:
Corinne Benakis
Email: Corinne.Benakis@med.uni-muenchen.de

In a new window | Download PPT
Figure 1: The gut-brain axis in acute brain injury. Acute brain injury induces microbiota dysbiosis and is associated with a stress response, intestinal barrier dysfunction, and immune imbalance. These phenomena are relayed to the brain via intestinal immune cell trafficking, increasing the neuroinflammatory response in a vicious cycle (Singh et al., 2016; Houlden et al., 2016; Stanley et al., 2016). Artificial change of the gut microbiota composition using antibiotics, reduces the development of the brain lesion in a mouse model of cerebral ischemia, improves sensory-motor functions caused by microbiota-dependent priming of dendritic cells inducing an expansion of regulatory T cells (Treg) suppressing γδT-IL17+ cells (Benakis et al., 2016). These findings suggest that the gut microbiota can influence development of the brain lesion via modulation of the microbiota–intestinal immune system interaction. Created with BioRender.com.

In a new window | Download PPT
Figure 2: Possible effects of microbiota-derived metabolites on acute brain injury. Dash arrows indicate that a direct link has not been described. Blue and red arrows indicate a favorable outcome or deleterious outcome, respectively. Created with BioRender.com.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 12939 | 116 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA