Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Targeting leukotriene biosynthesis to prevent atherosclerotic cardiovascular disease
Time:2023-12-01
Number:7096
Xiaomeng Wang1, Lohendran Baskaran2, Mark Chan3, William Boisvert4, Derek J Hausenloy1,5,6,7
Author Affiliations


- 1Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore.
- 2Department of Cardiology, National Heart Centre Singapore, Singapore.
- 3Department of Cardiology, National University Heart Centre, National University Health System, Singapore.
- 4Center for Cardiovascular Research, John A. Burns School of Medicine, University of Hawaii, USA.
- 5National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
- 6Yong Loo Lin Medical School, National University of Singapore, Singapore.
- 7The Hatter Cardiovascular Institute, University College London, London, UK.
Conditioning Medicine 2023. 6(2): 33-41.
Abstract
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and disability worldwide. As such, new treatments are needed to prevent the onset and progression of atherosclerosis to improve outcomes in patients with coronary, cerebrovascular, and peripheral arterial disease. In this regard, inflammation is known to be a critical driver of atherosclerosis formation and progression, thus it is a viable target for vascular protection in patients at risk of developing ASCVD. Leukotrienes, key pro-inflammatory lipid mediators derived from arachidonic acid, are associated with atheroma inflammation and progression. Genetic mutations in key components of the leukotriene synthesis pathway, such as 5-lipoxygenase (5-LO) and 5-lipoxygenase-activating protein (FLAP), are associated with an increased risk of cardiovascular disease, and pharmacological inhibition of 5-LO and FLAP has been reported to prevent atheroma formation in pre-clinical and early clinical studies. In this article, we provide an overview of these studies and highlight the therapeutic potential of targeting leukotriene synthesis to prevent atheroma inflammation and progression and improve outcomes in patients at risk of ASCVD.
Keywords: Atherosclerosis; Leukotrienes; Cardiovascular Diseases; 5-lipoxygenasE; 5-lipoxygenase-activating protein
Abstract
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and disability worldwide. As such, new treatments are needed to prevent the onset and progression of atherosclerosis to improve outcomes in patients with coronary, cerebrovascular, and peripheral arterial disease. In this regard, inflammation is known to be a critical driver of atherosclerosis formation and progression, thus it is a viable target for vascular protection in patients at risk of developing ASCVD. Leukotrienes, key pro-inflammatory lipid mediators derived from arachidonic acid, are associated with atheroma inflammation and progression. Genetic mutations in key components of the leukotriene synthesis pathway, such as 5-lipoxygenase (5-LO) and 5-lipoxygenase-activating protein (FLAP), are associated with an increased risk of cardiovascular disease, and pharmacological inhibition of 5-LO and FLAP has been reported to prevent atheroma formation in pre-clinical and early clinical studies. In this article, we provide an overview of these studies and highlight the therapeutic potential of targeting leukotriene synthesis to prevent atheroma inflammation and progression and improve outcomes in patients at risk of ASCVD.
Keywords: Atherosclerosis; Leukotrienes; Cardiovascular Diseases; 5-lipoxygenasE; 5-lipoxygenase-activating protein
Highlights
A major component of atherosclerotic cardiovascular disease involves aberrant inflammation that exacerbates the disease. In this paper, Mr. Wang and colleagues discuss the multi-facet features of the pro-inflammatory triggers leukotrienes, which closely approximate atheroma inflammation. In particular, the alteration in leukotrienes 5-lipoxygenase and 5-lipoxygenase-activating protein increases the risk of cardiovascular disease, whereas their pharmacological inhibition, in part recapitulating conditioning medicine strategies, robustly downregulates atheroma inflammation and improves cardiovascular function. Anti-inflammation therapies by sequestration of leukotrienes serves as novel conditioning medicine strategies for atherosclerotic cardiovascular disease characterized by deleterious inflammatory responses.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and disability worldwide (Kassebaum 2016; Wang et al., 2016), and new treatment strategies are needed to prevent the progression of atherosclerosis to improve outcomes of patients with coronary, cerebrovascular, and peripheral arterial disease. In this regard, inflammation is known to be a critical driver of atherosclerosis formation and progression (Libby et al., 2019), and therefore represents a viable target for vascular protection in patients at risk of developing ASCVD (Bäck et al., 2019).
Atherosclerosis is a complex inflammatory process involving asymmetric thickening of the arterial wall, triggered by the accumulation of cholesterol-containing low-density lipoprotein (LDL). The atherosclerotic lesion consists of lipids, connective tissue, and a variety of different types of cells (Hansson, 2005; Libby et al., 2011; Bäck and Hansson, 2015). Blood-borne leukocytes make up a significant portion of the atheroma cell population, with the remainder being vascular endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) (Hansson, 2005). Atherosclerosis is a condition characterized by inflammation, involving the infiltration of immune cells and the signaling of inflammatory cytokines within the atheroma (Jonasson et al., 1985; Hansson et al., 1989; Libby et al., 2002). Additionally, LDL particles possess the ability to activate both innate and adaptive immunity (Palinski et al., 1989; Stemme et al., 1995).
Despite optimal control of blood LDL cholesterol levels using lipid-lowering therapy such as statins, patients with acute myocardial infarction (AMI) still suffer from recurrent cardiovascular events indicative of residual cardiovascular risk, and this is primarily due to unresolved inflammation, also known as residual inflammatory risk (Bohula et al., 2018; Pradhan et al., 2018; Ridker, 2018). These findings have resulted in the development of anti-inflammatory strategies to prevent recurrent atherosclerotic cardiovascular events. In the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) trial, it was shown that targeting the interleukin-1β innate immunity pathway with canakinumab in previous AMI patients significantly reduced recurrent cardiovascular events compared to placebo, independent of lipid-level lowering (Ridker et al., 2017). Colchicine is an anti-inflammatory medication that is currently indicated for the treatment of gout, familial Mediterranean fever, and pericarditis (Perico et al., 1996; Pope and Tschopp, 2007; Dalbeth et al., 2014). In the COLCOT (Colchicine Cardiovascular Outcomes Trial), it was shown that targeting tubulin assembly, leukocytes chemotaxis, and phagocytosis with colchicine, reduced the risk of ischemic cardiovascular events in recent AMI patients (Dalbeth et al., 2014; Tardif et al., 2019). These studies underscored the importance of residual inflammatory risk. Other potential anti-inflammatory strategies include the inhibition of pro-inflammatory factors, such as pro-inflammatory cytokines and leukotrienes, a therapeutic approach that has been applied to other immune-mediated diseases, such as asthma. In this article, we highlight the role of the leukotriene synthesis pathway as a potential therapeutic target for reducing atherosclerosis and improving outcomes in patients at risk of developing ASCVD.
The leukotriene synthesis pathway
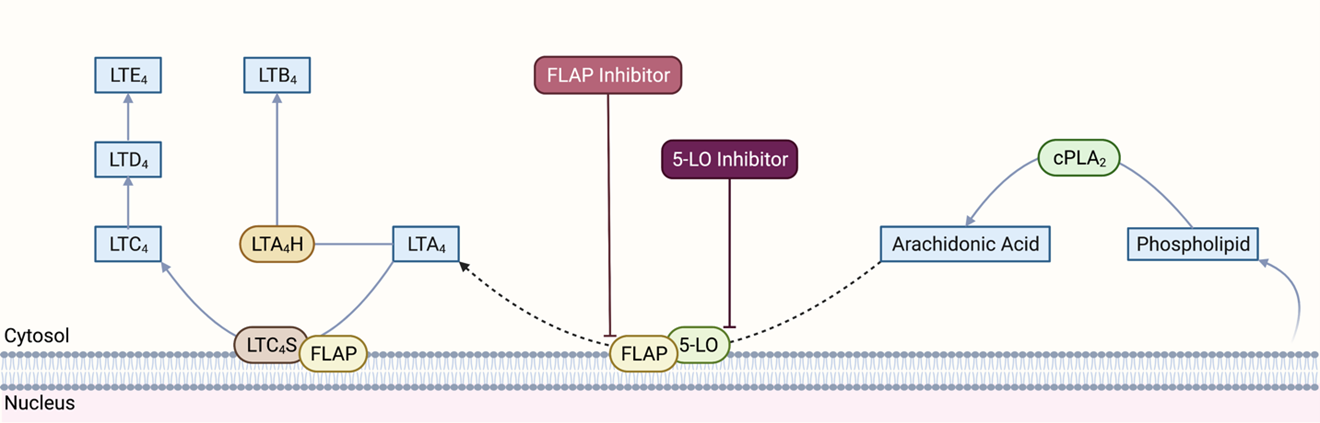
Leukotrienes are a family of pro-inflammatory lipid mediators that play an essential role in immune-mediated diseases such as asthma, rhinitis, ASCVDs, and cancer (De Caterina and Zampolli, 2004; Bäck, 2008; Bäck, 2009; Sampson, 2009; Wang and DuBois, 2010; Gür et al., 2018). When triggered by inflammatory, allergic, or immune signals, activated cytosolic phospholipase A2 (cPLA2) converts phospholipids from the cellular membrane to arachidonic acid (AA) (Figure 1). AA is first oxygenated to 5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE) and then dehydrated into an unstable intermediate leukotriene, leukotriene A4 (LTA4), by 5-lipoxygenase (5-LO). The flux of this reaction depends on the activity of 5-LO, which may be influenced by phosphorylation, calcium and magnesium ion concentrations, phosphatidylcholine activation status, and other protein-protein interactions (Hill et al., 1992; Radmark et al., 2015; Gerstmeier et al., 2016). In this process, membrane-associated 5-lipoxygenase-activating protein (FLAP) facilitates 5-LO docking in the nuclear membrane, where it selectively transfers AA to 5-LO, and enhances sequential oxygenation-dehydration reactions (Young, 1999; Pettersen et al., 2015). However, it is important to note that how FLAP achieves this is still not completely understood.
In a new window | Download PPT
Figure 1. Leukotriene synthesis pathway. cPLA2: cytosolic phospholipase A2, 5-LO: 5-lipoxygenase; FLAP: 5-lipoxygenase-activating protein; LTA4: leukotriene A4; LTB4: leukotriene B4; LTC4: leukotriene C4; LTD4: leukotriene D4; LTE4: leukotriene E4; LTA4H: leukotriene A4 hydrolase; LTC4S: leukotriene C4 synthase. Created with BioRender.com.
LTA4 may be used for the biosynthesis of LTB4 or cysteinyl leukotrienes (LTC4, LTD4 and LTE4) (Evans et al., 2008; Pettersen et al., 2015; Gür et al., 2018). LTA4 hydrolase (LTA4H), a Zn-associated epoxide hydrolase, produces LTB4 from LTA4. Nuclear membrane associated LTC4 synthase (LTC4S) conjugates LTA4 with glutathione to produce LTC4, the first of three cysteinyl leukotrienes. Specific transport proteins export LTB4 and LTC4 out of the cell. In the extracellular space, LTC4 may be first converted to LTD4 by glutamyl transpeptidase and then to LTE4 by dipeptidase. Notably, although 5-LO and FLAP are primarily expressed in a subset of leukocytes, such as monocytes and macrophages, some other cell types, such as ECs and VSMCs, can still produce LTB4 and cysteinyl leukotrienes due to the intercellular transfer of LTA4 (Folco and Murphy, 2006). Recent studies have also identified homology between FLAP and LTC4 synthase and the existence of a FLAP/LTC4 synthase gene family. However, the significance of such homology is still not fully understood (Hatzelmann et al., 1994; Lam et al., 1994; Byrum et al., 1997).
LTB4 is a pro-inflammatory mediator that induces neutrophil chemotaxis to sites of inflammation where it exerts its biological function by binding to two G-protein coupled receptors (GPCRs), BLT1 and BLT2. LTB4 may also activate leukocytes, inhibit neutrophil apoptosis, and increase adhesion molecule expression (Afonso et al., 2012). Cysteinyl leukotrienes primarily mediate vasoconstriction and blood vessel permeability and have pro-inflammatory effects. These leukotrienes exert their actions through two sets of GPCRs, CysLT1, and CysLT2 receptors. GPR17 receptors, which are mostly expressed in organs undergoing ischemic damage, also have a high affinity for cysteinyl leukotrienes (Ciana et al., 2006).
5-LO as a target for vascular protection
The 5-LO pathway has long been a focus of research because leukotrienes play vital roles in acute or chronic inflammatory disease pathogenesis. 5-LO pathway-targeting drug development has focused on respiratory diseases, such as asthma, because cysteinyl leukotrienes mediate bronchoconstriction and induce pulmonary inflammation (Pettersen et al., 2015). The role of leukotrienes as mediators of atherosclerosis in cardiovascular disease has attracted increasing interest. Early studies have found that coronary arteries produce cysteinyl leukotrienes and coronary arteries with atheroma have a higher response to these cysteinyl leukotrienes due to an increased expression of cysteinyl leukotriene receptors (Piomelli et al., 1987; Allen et al., 1998). These studies also correlated serum LTB4 levels with leukocyte content in human carotid plaques, implicating a role for leukotrienes in atherosclerosis formation and progression (De Caterina et al., 1988). The expression and activity of the 5-LO pathway have been associated with atheroma progression and instability, which causes plaque rupture, thrombosis, blockage of coronary vessels, and eventually AMI (Mehrabian et al., 2002; Spanbroek et al., 2003; Cipollone et al., 2005; Qiu et al., 2006). Clinical studies have also found that elevated serum LTB4 and urine LTE4 levels predict acute coronary syndrome (ACS) risk in patients (Carry et al., 1992; Allen et al., 1993; Sanchez-Galan et al., 2009; Guo-ping et al., 2012).
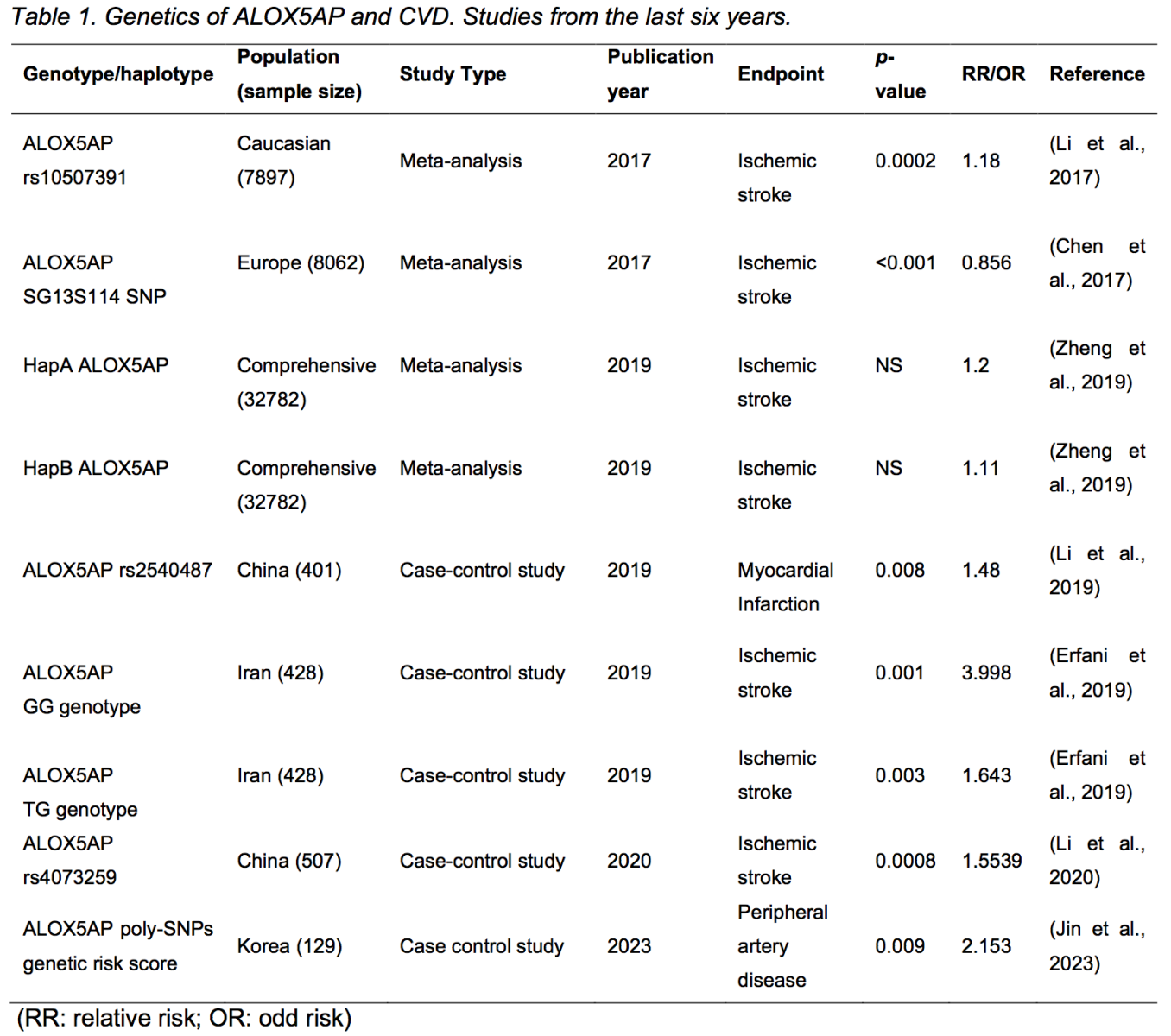
Genetic studies have corroborated the link between the 5-LO pathway and cardiovascular disease. The HapK haplotype of LTA4H, the gene encoding LTA4H, has been associated with an increased risk of AMI and other cardiovascular diseases in Icelandic and USA patients (Helgadottir et al., 2006). Helgadottir et al. (2006) also found that the HapK haplotype of LTA4H caused a gain of LTA4H function, as researchers observed a positive correlation between the HapK haplotype and LTB4 production in granulocytes collected from carriers and non-carriers. A promoter polymorphism of the LTC4S gene has been linked to increased coronary artery calcium and arterial intima-media thickness, a surrogate marker for atherosclerotic plaque burden, in a study with young females from Muscatine, Iowa (Iovannisci et al., 2007). Genotypes of the ALOX5 promotor were also correlated with carotid artery intima-media thickness and serum markers of inflammation in a study with a middle-aged population from Los Angeles, California (Dwyer et al., 2004). However, genome-wide association studies with ALOX5AP, which encodes FLAP, had controversial results. In 2004, Helgadottir et al. (2004) found that specific ALOX5AP haplotypes predicted increased risk of AMI and stroke in Icelandic and British populations, while such haplotypes were not associated with increased infarction risk in a later study with populations from the United States, Germany, and Japan (Funk, 2005; Lõhmussaar et al., 2005; Kaushal et al., 2007). To further reveal the association between ALOX5AP variations and cardiovascular risk, more genome-wide association studies have been performed and are reviewed elsewhere (Funk, 2005; Evans et al., 2008; Bäck, 2009). Recent genome association studies and meta-analyses mostly agree that specific haplotypes, genotypes, or polymorphisms of ALOX5AP are associated with altered risks of cardiovascular conditions, including AMI, stroke, and peripheral artery disease (Table 1). In their study, Helgadottir et al. (2004) discovered that disease-associated variants of ALOX5AP increased the response of FLAP to pro-inflammatory factors, thereby intensifying the inflammatory response of the 5-LO pathway. However, there remains a limited number of studies exploring the specific impact of these ALOX5AP variants on FLAP function. Further large-scale studies are necessary to verify the functional implications of disease-associated variants affecting FLAP and other enzymes within the 5-LO pathway.
Pre-clinical studies targeting 5-LO for vascular protection
In pre-clinical models of cardiovascular disease, inhibiting leukotriene production genetically or pharmacologically attenuates atherosclerosis disease progression and ameliorates the consequences (Lepran and Lefer, 1985; Hock et al., 1992; Poeckel and Funk, 2010). Genetic knockout of 5-LO has been shown to reduce atherosclerosis development and cholate-induced abdominal aneurysm in LDL receptor knockout mouse model (Mehrabian et al., 2002; Zhao et al., 2004). Oral administration of 5-LO inhibitors in atherosclerosis-prone (LDLR-/- or ApoE-/-) mouse models has also yielded beneficial effects (Whatling et al., 2007; Back, 2009; Cao et al., 2009). More follow-up studies are developing novel 5-LO inhibitors and investigating the biological effects, and are reviewed elsewhere (Bäck, 2009; Sinha et al., 2019; Orafaie et al., 2020).
Animal studies have shown that some other current medications that target the 5-LO pathway may also have the potential to attenuate vascular inflammation in atherosclerotic diseases. Licofelone is a potent anti-inflammatory agent targeting lipoxygenase and cyclooxygenase (COX). This medicine was first designed to treat osteoarthritis, but Vidal et al. (2007) revealed its anti-inflammatory effect in the atherosclerotic rabbit model (Alvaro-Gracia, 2004). In the lesion region, licofelone reduced intima thickening, macrophage infiltration, the expression of pro-inflammatory cytokines (monocyte chemoattractant protein-1 and nuclear factor kappa-light-chain-enhancer of activated B cells [NF-κB], and the 5-LO enzyme. Licofelone administration also inhibited LTB4 synthesis in neutrophils of atherosclerotic rabbits (Vidal et al., 2007). Another 5-LO/COX dual inhibitor, zileuton, is a common anti-inflammatory agent for respiratory diseases, such as asthma (Wenzel and Kamada, 1996). Abueid et al. (2017) administered zileuton to rats with induced myocardial ischemia-reperfusion injury and found that zileuton treatment could blunt tissue injury and reduce NF-κB release and cardiomyocyte apoptosis in the injury region. However, zileuton treatment failed to affect serum pro-inflammatory tumor necrosis factor-α (TNF-α) levels among injured rats (Abueid et al., 2017). Besides anti-inflammatory agents, statin, a commonly used lipid-lowering agent in patients with atherosclerotic diseases, was also found to target the 5-LO pathway. An in vitro study with isolated adipocytes showed that acute treatment (6 hours) with high-dose atorvastatin reduced the expression of 5-LO and inflammatory biomarkers, suggesting an inhibitory effect on this pathway (Wang et al., 2014).
Besides synthetic 5-LO inhibitors, many natural substances and herbal medications also exert anti-inflammatory effects by targeting 5-LO. Caffeic acid phenethyl ester (CAPE) is a naturally occurring compound exhibiting antioxidant and anti-inflammatory effects (Sud'Ina et al., 1993; Mirzoeva and Calder, 1996; Michaluart et al., 1999; Gokalp et al., 2006; Parlakpinar et al., 2006). Boudreau et al. (2012) revealed that such anti-inflammatory potency lies in the inhibitory effect of 5-LO activity and AA synthesis. Researchers also suggested that the CAPE structure could serve as a framework for future design of leukotriene synthesis inhibitors (Boudreau et al., 2012). Hassan et al. (2014) found that administration of CAPE to both insulin-resistant rats and insulin-deficient rats attenuated blood pressure increase, reduced circulatory pro-inflammatory cytokines, and reduced aortic collagen deposition, suggesting that CAPE could offset diabetes-associated atherosclerotic changes. Researchers also found that CAPE prevented the development of hyperinsulinemia in insulin-resistant rats (Hassan et al., 2014). The perennial herb Plectranthus zeylanicus Benth (a plant that is locally known as Iruveriya) is extensively used in South Asian ethnomedicine as an anti-inflammatory remedy, and Napagoda et al. (2014) have found that extracts of Plectranthus zeylanicus with n-hexane or dichloromethane could potently inhibit isolated human recombinant 5-LO and 5-LO in stimulated human neutrophils. Flavocoxid, a medical food extracted from Scutellaria baicalensis (Chinese skullcap) and Acacia catechu (black catechu), has been found to exert anti-inflammatory and antioxidant effects by targeting 5-LO and COX enzyme (LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, 2012-; Bitto et al., 2014). Flavocoxid was a previously FDA-approved medical food to treat chronic osteoarthritis, but the approval was withdrawn in 2017 (LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, 2012-). El-Sheakh et al. (2015) found that in high-cholesterol-fed rabbits, flavocoxid treatment reduced the expression and release of inflammation biomarkers (C-reactive protein [CRP], TNF-α, NF-κB), and attenuated the elevation in the tunica intima-media ratio in the aorta, suggesting that flavocoxid treatment could prevent the inflammation and vascular dysfunction induced by a high-cholesterol diet. Moderate consumption of wine is associated with reduced incidence of coronary artery disease, and Kutil et al. (2014) have found that red and white wines inhibit the activity of isolated recombinant 5-LO, indicating a potential mechanistic explanation for wine consumption beneficial effects. They also identified piceatannol, luteolin, quercetin, and myricetin as the components in wine exerting the most potent 5-LO inhibiting effects (St Leger et al., 1979; Renaud and de Lorgeril, 1992; Kutil et al., 2014). However, although these studies demonstrated that these naturally occurring substances have a 5-LO inhibitory effect, further studies are needed to confirm their therapeutic efficacy in preventing atherosclerosis.
Clinical studies targeting 5-LO for vascular protection
Atreleuton (VIA-2291, Tallikut Pharmaceuticals, United States) is the first, and only 5-LO inhibitor whose therapeutic efficacy against atherosclerotic disease has been tested in randomized clinical trials. This drug was initially designed to treat asthma due to its potency for inhibiting LTB4 and LTE4 synthesis (Lehnigk et al., 1998). In 2010, Tardif et al. (2010) published their phase II randomized controlled trial involving 191 patients with recent ACS and found that atreleuton treatment significantly reduced serum LTB4 and urine LTE4 in a dose-dependent fashion without any adverse effects. A follow-up serial computed tomographic (CT) angiography study revealed that 6 months of atreleuton treatment reduced atheroma progression and the development of new atheroma in the treatment group when compared to placebo (Matsumoto et al., 2017). A further post-hoc study reported that after controlling for traditional cardiovascular risk factors (age, gender, body mass index, dyslipidemia, smoking, and demographics), atreleuton treatment yielded a significant decrease in epicardial adipose tissue (EAT) and pericardial adipose tissue (PAT) in patients in the treatment groups vs. placebo. These authors also found that the reduction in EAT volume correlated with the reduction in total atherosclerotic plaque volume across all atreleuton treatment groups, suggesting that EAT volume could be a predictor for future cardiovascular diseases (Almeida et al., 2020).
In 2015, Gaztanaga et al. (2015) published a second phase II randomized trial involving 52 recent ACS patients to investigate the effect of atreleuton on vascular inflammation. In the trial, subjects received daily 100 mg atreleuton for 24 weeks. Like Tardif et al. (2010), Gaztanaga et al. (2015) found that atreleuton treatment reduced leukotriene levels. However, Gaztanaga et al. (2015) found that the treatment failed to reduce vascular inflammation (by FDG-PET) and other serum inflammatory markers, such as high sensitivity CRP. In the Tardif et al. study (2010), patients receiving 100 mg atreleuton showed a significant decrease in high sensitivity CRP at 24 weeks’ treatment. One potential reason for such a discrepancy is that the Tardif et al. trial (2010) had a bigger sample size (n = 48 for the placebo and n = 38 for the 100 mg treatment group) than the Gaztanaga et al. trial, (2015), allowing for greater statistical power.
FLAP as a target for vascular protection
FLAP is also a promising anti-inflammatory target in the leukotriene synthesis pathway, and FLAP inhibitors appear to be more efficacious than 5-LO inhibitors, potentially due to
the lower turnover of FLAP in leukotriene-producing cells (Evans et al., 2008). In 2005, Hakonarson et al. (2005) conducted a randomized, prospective, placebo-controlled, crossover phase II trial of DG-031 (Bayer Health Care AG, Leverkusen, Germany), a potent FLAP inhibitor, in AMI patients who carried at-risk variants in the ALOX5AP gene or the LTA4H gene (Hakonarson et al., 2005). High-dose DG-031 (750 mg/day) treatment reduced the production of LTB4 (by 26%) and myeloperoxidase (by 12%) and reduced CRP levels (by 25%) when compared to placebo, suggesting that DG-031 treatment significantly suppressed inflammatory biomarkers associated with increased risk of AMI events. However, the phase III trial of DG-031 (ClinicalTrials.gov identifier NCT00353067) was suspended in 2006 due to unexpected formulation issues. Also, in 2006, Jawien et al. (2006) demonstrated that MK-886 (Merck, Rahway, NJ), another potent FLAP inhibitor, could prevent new aortic atheroma development, attenuate aortic root dilation, and reduce atheroma macrophage content, without affecting blood cholesterol levels, in apolipoprotein E/low density lipoprotein receptor (apoE/LDLR) double knockout (DKO) mouse model.
On this background, the development of new FLAP inhibitors has become a new research focus, and the chemical evolution of FLAP inhibiting molecules has been recently reviewed (Pettersen et al., 2015; Gür et al., 2018). In 2007, Merck announced the completion of a phase II trial (ClinicalTrials.gov identifier NCT00421278) of MK-0633, a FLAP inhibitor previously developed for treating asthma and chronic obstructive pulmonary disease in atherosclerotic patients, but no results were announced. By 2009, Merck applied for several FLAP inhibitor patents, which involved two potent FLAP-inhibiting molecules with novel structures (Tavridou and Manolopoulos, 2009). In 2010, Bain et al. (2010) announced the result of the phase I trial of AM103, a novel FLAP inhibitor developed for respiratory and cardiovascular disease, showing good tolerance, but no further study has been announced.
AZD5718 (Astra Zeneca, Cambridge, United Kingdom) is the most recent FLAP inhibitor developed for atherosclerotic diseases. Pre-clinical studies have demonstrated a potent FLAP inhibitory effect and a good safety profile in animal studies (Lemurell et al., 2019; Pettersen et al., 2019). The first-in-human study of AZD5718 was a daily oral single and multiple ascending dosing study in 96 healthy male subjects, and no safety or tolerability issues were reported (Ericsson et al., 2018). AZD5718 was reported to be absorbed rapidly, with a mean terminal half-life of 10–12 h, and it reduced serum LTB4 and urine LTE4 levels, demonstrating its therapeutic potential. These findings were later confirmed in a follow-up phase I study in 32 healthy Japanese male subjects (Knöchel et al., 2021). In post hoc pharmacodynamic simulations, AZD5718 was shown to reduce plasma LTB4 levels by >90%, further confirming its potency as an anti-inflammatory agent (Ericsson et al., 2020). AZD5718 was investigated in the multicenter phase IIa randomized controlled FLAVOUR trial, which evaluated the safety and efficacy of AZD5718 in recent AMI patients (1-4 weeks) (Prescott et al., 2020). The primary efficacy outcome of FLAVOUR was the change in urine LTE4 levels, and the secondary efficacy outcome was improvement in coronary microvascular function, as measured by transthoracic color Doppler-assisted coronary flow velocity reserve (CFVR) (Prescott et al., 2020). In the FLAVOUR trial, mean urine LTE4 levels decreased in the AZD5718 treatment groups but not in the placebo group, with statistically significant reductions at 4 and 12 weeks. There were no significant or clinically meaningful changes in CFVR observed for AZD5718 vs. placebo (Prescott et al., 2022). The ongoing PASSIVATE multicenter randomized controlled clinical trial is investigating the efficacy of AZD5718 in preventing coronary atheroma progression assessed by CTCA in recent AMI patients (NCT04601467).
Conclusions
In summary, leukotrienes are critical drivers of inflammation in atherosclerotic cardiovascular diseases and inhibiting the leukotriene biosynthesis pathway by inhibiting 5-LO and FLAP provides a therapeutic strategy for preventing atherosclerosis formation and progression. Clinical studies are ongoing to determine whether this therapeutic approach can improve outcomes in patients with ASCVD.
Conflicts of interest
XMW, WB, LB and DJH declare no conflict of interest. MC has received funding from Astra Zeneca for the PASSIVATE trial. DJH is an Editor for Conditioning Medicine, and he has not participated at any level in the editorial review of this manuscript.
Acknowledgements
This project was funded by NIH grant R01HL81863 to WAB. DJH is supported by the Duke-NUS Signature Research Programme funded by the Ministry of Health, Singapore Ministry of Health’s National Medical Research Council under its Singapore Translational Research Investigator Award (MOH-STaR21jun-0003), Centre Grant scheme (NMRC CG21APR1006), and Collaborative Centre Grant scheme (NMRC/CG21APRC006). This article is based upon work supported by the National Research Foundation Competitive Research Program (NRF CRP25-2020RS-0001) and COST Action EU-CARDIOPROTECTION IG16225 supported by COST (European Cooperation in Science and Technology). Mark Y. Chan receives salary support and research grant support from a clinician scientist award – senior investigator (NMRC MOH-000280-00), centre grant (NMRC CG21APR1008), Collaborative Centre Grant scheme (NMRC/CG21APRC006), MOH Health Services Research Program (MOH MH70:70/1-2017). The Passivation of Vulnerable Plaque with AZD5718 in Acute Coronary Syndrome (PASSIVATE) trial is supported by an Externally Sponsored Clinical Research (ESCR) grant from Astra-Zeneca (Cambridge, UK).
References
Xiaomeng Wang1
1Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore.
Lohendran Baskaran2
2Department of Cardiology, National Heart Centre Singapore, Singapore.
Mark Chan3
3Department of Cardiology, National University Heart Centre, National University Health System, Singapore.
William Boisvert4
4Center for Cardiovascular Research, John A. Burns School of Medicine, University of Hawaii, USA.
Derek J Hausenloy1,5-7#
1Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore. 5National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 6Yong Loo Lin Medical School, National University of Singapore, Singapore. 7The Hatter Cardiovascular Institute, University College London, London, UK.
Corresponding author:
Prof Derek J. Hausenloy
Emil: derek.hausenloy@duke-nus.edu.sg

In a new window | Download PPT
Figure 1. Leukotriene synthesis pathway. cPLA2: cytosolic phospholipase A2, 5-LO: 5-lipoxygenase; FLAP: 5-lipoxygenase-activating protein; LTA4: leukotriene A4; LTB4: leukotriene B4; LTC4: leukotriene C4; LTD4: leukotriene D4; LTE4: leukotriene E4; LTA4H: leukotriene A4 hydrolase; LTC4S: leukotriene C4 synthase. Created with BioRender.com.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7096 | 11 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA