Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Cardioprotective role of insulin on long QT-interval via recoveries in K+-channel currents in advanced age
Time:2022-08-01
Number:6256
Belma Turan1
Author Affiliations


- 1Department of Biophysics, Lokman Hekim University Faculty of Medicine, Ankara, Turkey.
Conditioning Medicine 2022. 5(2):51-58.
Abstract
International consensus recognizes the impact of the aging process on the development of left ventricular hypertrophy in the heart. Both experimental and clinical data emphasize the intersection between aging and cardiovascular disease, which include age-associated co-morbidities such as metabolic syndrome, obesity, and diabetes that enhance the risk of developing cardiovascular disease. Indeed, cardiometabolic disturbances, including insulin resistance, contribute to aging-associated cardiac insufficiency and/or dysfunction. Cardiac aging is an intrinsic process accompanied by molecular and cellular changes (cardiac electrophysiological remodeling) mainly characterized by a long QT-interval, increased heart rate, and depressed cardiac output, as well as prolonged action potentials, altered sarcolemmal ionic currents and intracellular Ca2+-regulation at the cellular levels. Previous studies emphasize the benefits of insulin regulation as a critical component of pharmacotherapy after myocardial injury. Given the relationship between impaired insulin signaling and depression of various voltage-dependent K+-channels, which in turn contribute to prolonged action potentials and long-QT in the heart, insulin treatment to restore proper cellular signaling may become an emerging new field for treatiing age-related heart dysfunction. This review-article focuses on the molecular mechanisms linking insulin resistance, heart dysfunction, and advanced age in mammalians, including recent experimental results associated with the possible contribution of a right-shift of the voltage-dependency of tetrodotoxin-sensitive Na+-currents in ventricular cardiomyocytes from aged rat hearts, as well as the beneficial effects of insulin treatment in aged-rats on their cardiovascular function. Hopefully, this document will encourage scientists and clinicians in this field to design new and more effective mechanism-based insulin-like agents to improve myocardial performance in aged humans.
Keywords: Insulin, Aging, Electrical remodeling, Heart dusfunction, Mitochondria
Abstract
International consensus recognizes the impact of the aging process on the development of left ventricular hypertrophy in the heart. Both experimental and clinical data emphasize the intersection between aging and cardiovascular disease, which include age-associated co-morbidities such as metabolic syndrome, obesity, and diabetes that enhance the risk of developing cardiovascular disease. Indeed, cardiometabolic disturbances, including insulin resistance, contribute to aging-associated cardiac insufficiency and/or dysfunction. Cardiac aging is an intrinsic process accompanied by molecular and cellular changes (cardiac electrophysiological remodeling) mainly characterized by a long QT-interval, increased heart rate, and depressed cardiac output, as well as prolonged action potentials, altered sarcolemmal ionic currents and intracellular Ca2+-regulation at the cellular levels. Previous studies emphasize the benefits of insulin regulation as a critical component of pharmacotherapy after myocardial injury. Given the relationship between impaired insulin signaling and depression of various voltage-dependent K+-channels, which in turn contribute to prolonged action potentials and long-QT in the heart, insulin treatment to restore proper cellular signaling may become an emerging new field for treatiing age-related heart dysfunction. This review-article focuses on the molecular mechanisms linking insulin resistance, heart dysfunction, and advanced age in mammalians, including recent experimental results associated with the possible contribution of a right-shift of the voltage-dependency of tetrodotoxin-sensitive Na+-currents in ventricular cardiomyocytes from aged rat hearts, as well as the beneficial effects of insulin treatment in aged-rats on their cardiovascular function. Hopefully, this document will encourage scientists and clinicians in this field to design new and more effective mechanism-based insulin-like agents to improve myocardial performance in aged humans.
Keywords: Insulin, Aging, Electrical remodeling, Heart dusfunction, Mitochondria
Introduction
Changes in individual cells in all organs of the body lead to progressive deterioration in their functions and structures that underlie the process of human aging. Aging is a major risk factor for cardiovascular diseases, the leading cause of death worldwide (Lakatta et al., 2001). The most common change in the cardiovascular system (CVS) is stiffening of the arteries and other blood vessels, causing the heart to work harder, which leads to further remodeling of the heart muscles to adjust to the increased workload (Waring et al., 2014). After such changes, the heart rate at rest will stay about the same, but will fail to increase during activities as it once did. These changes increase the risk of high blood pressure (hypertension) and other cardiovascular alterations.
Cardiovascular diseases are the leading cause of death in most countries. Although it has received the least public attention, aging is by far the dominant risk factor for the development of cardiovascular diseases, as the prevalence of cardiovascular diseases increases dramatically with increasing age (Lakatta et al., 2001). Aging hearts, in general, show a progressive decline in their structure, function, and metabolism (Ruiz-Meana et al., 2020; Strait and Lakatta, 2012). In large part this is due to impairment in electrical properties of cardiomyocytes, as well as the changes in the conduction of electrical signals among myocytes. In addition, aged hearts show impairments in redox status, metabolic flexibility, and organelle dynamics (Lesnefsky et al., 2016). Most clinical observations point out the parallelism between the occurrence of inevitable functional decline with time in the heart and induction of insulin resistance (Chason et al., 2018). Both clinical and experimental data mentioned that cardiac aging is mainly characterized by a long QT-interval in electrocardiograms (ECGs), a reduction in the maximum heart rate, and a decrease in the contractile activity, at most, through cardiometabolic disturbances (Lakatta et al., 2001). Cardiometabolic disturbances are mainly characterized by insulin resistance and the appearance of glucose intolerance, which are factors that underlie the impairment in electrophysiological activities, and myocardial cell death (Boudina, 2013; Olgar, et al. 2018; Olgar et al., 2020a; Olgar et al., 2020b; van Noord et al., 2010). Experimental animal studies, with either cardiac-specific insulin receptor deletion or insulin application, have shown the important role of insulin on the conduction velocity of electrical signaling and ventricular repolarization in the heart. Correspondingly, insulin application provided significant cardioprotection in the rat heart with not only metabolic syndrome but also advanced age (Olgar et al., 2021a). Most importantly, these cardioprotective effects of insulin are due to its benefits on the depressed expression and function of voltage-dependent K+-channels in left ventricular cardiomyocytes (Bertrand et al., 2008; Lopez-Izquierdo et al., 2014; Olgar et al., 2020a; Olgar et al., 2020b).
Most experimental findings support the hypothesis that lack of insulin signaling can provide cardioprotection in a reshape action potential of left ventricular cardiomyocytes which further leads to abnormal ventricular repolarization in the advanced heart. Therefore, this review focuses particularly on the molecular mechanisms linking insulin resistance, heart dysfunction and advanced age in mammalians, including recent experimental results showing a possible contribution of a right-shift of the voltage-dependency of tetrodotoxin (TTX)-sensitive Na+-currents in ventricular cardiomyocytes from aged rat hearts. The beneficial effects of insulin treatment on cardiovascular function in aged-rats will also be discussed, including original in vivo and in vitro work examining the actions of insulin in the aged rat hearts will be Therefore, this review will open the door to design new and more effective mechanism-based insulin-like agents to improve myocardial performance in elderly humans.
Cardiac ventricular electrophysiological remodeling in the elderly mammalian heart
Biological aging is the greatest risk factor for cardiovascular-related morbidity and mortality (Mozaffarian et al., 2016). Normal physiological remodeling in healthy aging hearts mainly includes changes in electrical conduction among tissues, valve function, large and small coronary vessels, and contractile activity. These age-related physiological alterations can in turn be exaggerated stimuli for tissue remodeling during pathophysiological states. There are marked structural remodeling in aging hearts, including atrial and ventricular fibrosis, which can further promote progression to ventricular arrhythmia, heart failure, and sudden cardiac death in aged rabbit heart (Lin et al., 2018; Stuart et al., 2018). Similar structural changes have been determined in the aged rat heart together with marked fragmentation in mitochondria in isolated ventricular cardiomyocytes (Olgar et al., 2020a; Olgar et al., 2021a). Among these changes, passive ventricular remodeling is also defined by the process of molecular remodeling of gap junctions, that can affect cell-to-cell propagation of the electrical impulse, inducing re-modification in the excitability (Kessler et al., 2014; Xie et al., 2013).
Cardiomyocytes isolated from the left ventricular part of the aged animal heart showed marked myocyte modification characterized by a high level of oxidative stress, cell loss and hypertrophy, apoptosis, and autophagy (Sheydina et al., 2011; Zhang et al., 2015). Aging also prolongs the duration of action potentials (APs) of cardiomyocytes isolated from the left ventricle, through alterations in the electrophysiological characteristics of the ionic currents (Olgar et al., 2020a; Olgar et al., 2020b). Ventricular cardiomyocytes undergoing age-associated electrophysiological remodeling typically exhibit a prolonged AP duration that has been attributed to a decrease in voltage-dependent outward K+-channel currents (Janczewski, Spurgeon, & Lakatta, 2002) together with significant increases in the Na+/K+-pump current, Na+/Ca2+-exchanger current, and intracellular levels of Na+, Ca2+ and H+, parallel to long QT-interval in the surface ECGs (Olgar et al., 2020a; Olgar et al., 2020b). Although there were no significant changes in the peak amplitude of the voltage-dependent Na+-channel currents in the left ventricular cardiomyocytes from the aged rat hearts, the characteristic of the frequency distribution of APs seems to be different in the aged rats (24-mo-old) compared to the adult rats (6-mo-old), including a marked shift with the positive potentials (Fig. 1). This right-side shift of the histograms provides strong evidence of an increase in late Na+-channel currents in the ventricular myocytes. Besides this preliminary observation, other studies demonstrated that neither the density nor the expression level of voltage-dependent Na+-channels is affected by aging (Baba et al., 2006), whereas a significant increase is observed in the late Na+-channel currents in the aged ventricular cardiomyocytes (Signore et al., 2015).
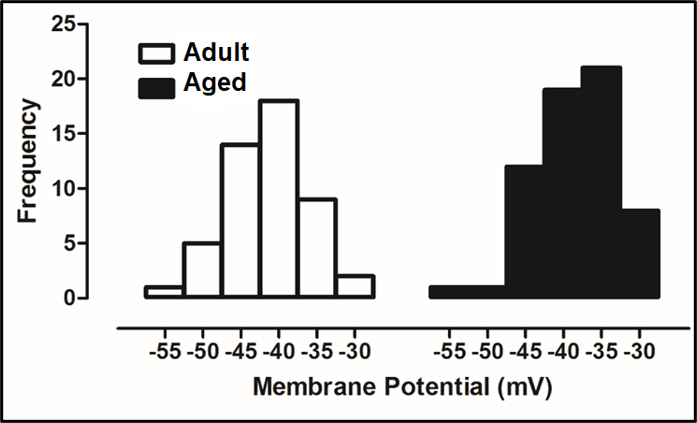
In a new window | Download PPT
Figure 1: Whole-cell TTX-sensitive voltage-dependent Na+-channel currents were recorded in freshly isolated ventricular cardiomyocytes from male Wistar rats with an age of 24-mo-old (Aged group) and age of 8-mo-old (Adult group) as described, previously (Bilginoglu et al., 2013). Freshly cardiomyocyte isolation was performed by using the enzymatic method, as described, previously (Olgar et al., 2021). For comparison, all recorded currents were divided by the cell membrane capacitance to present them as current density (in pA/pF). The frequency of the maximum amplitude variation with membrane potentials is presented as histograms for adult (left) and aged (right) groups, respectively. The frequency of the maximum amplitude of the aged group did shift to the right (positive potentials) starting from -40 mV in comparison to the adult group, implying the existence of late Na+-currents.
Moreover, it has been demonstrated that aging was associated with depression of the sarcoplasmic reticulum (SR) Ca2+-level and intracellular Ca2+ changes under electrical stimulation in cardiomyocytes from not only aged humans but also other aged animals (Herraiz-Martínez et al., 2015; Olgar et al., 2020a; Olgar et al., 2020b). These alterations are also closely associated with a progressive decline in contractile function with aging even under physiological conditions.
Cardioprotective action of insulin in the aged mammalian heart
There is a close relationship between increased life span and the development of at least one chronic disease, which directly impacts the overall health of the elderly population. Although intrinsic cardiac aging is an independent risk factor for the development of heart insufficiency/dysfunction, its impact is confounded by various age-relted risk factors, including insulin resistance (Lakatta, 2000). The involvement of the insulin-signaling pathway in life span extension is widely known, and as the most conserved signaling pathway, it also has an importnt impact on cardiac physiological aging (Inuzuka et al., 2009; Kenyon, 2005; Wessells et al., 2004). So, insulin may attenuate cardiac aging by affecting cardiac insulin signaling, although the underlying mechanisms are not well understood. A few studies have examined the impact of a reduction in insulin/IGF-signaling on aging-associated cardiac alterations and the role of its alleviation on the preservation of cardiac performance (Barzilai and Ferrucci, 2012; Yang et al., 2017; Yu et al., 2011). The early studies on the cardiovascular actions of insulin in humans have determined there is a correlation between insulin resistance and both reduction in cardiac index and stroke volume without a change in heart rate (Baron et al., 1990). The findings from these early studies strongly emphasized the in vivo role of insulin as an endocrine regulator of cardiovascular physiology through both glucose uptake and hemodynamic regulation.
Insulin is an anabolic peptide hormone that plays a vital role in the regulation of human metabolism during the lifespan as well as it actions on a specific cell membrane receptor. Although it is widely accepted to play a key role in glucose-homeostasis, it is now appreciated how insulin can target different signaling pathways and that it has a wide-range of pleiotropic roles in mammalian cells. Insulin is known to be a main energy-storage and metabolism regulator, and it has critical but very complex effects in basic organs such as liver, muscle, brain, and adipose tissue (Fig. 2, left). As can be seen in the proposed schema, insulin targets several systems through its receptor and affects multiple physiological processes in the organism, such as attenuations of glucose metabolism/synthesis, mitochondrial dysfunction, and lipogenesis in muscle, adipose tissue, and liver, whereas it decreases glucose and lipoprotein productions in the brain. Also, insulin through its receptor plays critical roles in intracellular metabolic pathways directly (i.e. insulin signaling pathways) or indirectly (via other metabolic pathways). As can be seen in Fig. 2 (right), intracellular pathways can be summarized as increases in glucose uptake, synthesis of glucogen, protein, and DNA, and attenuation of gene expression and lipogenesis with decreases mainly in mitochondrial dysfunction and related facors, glucogenesis, apoptosis, and autophagy.
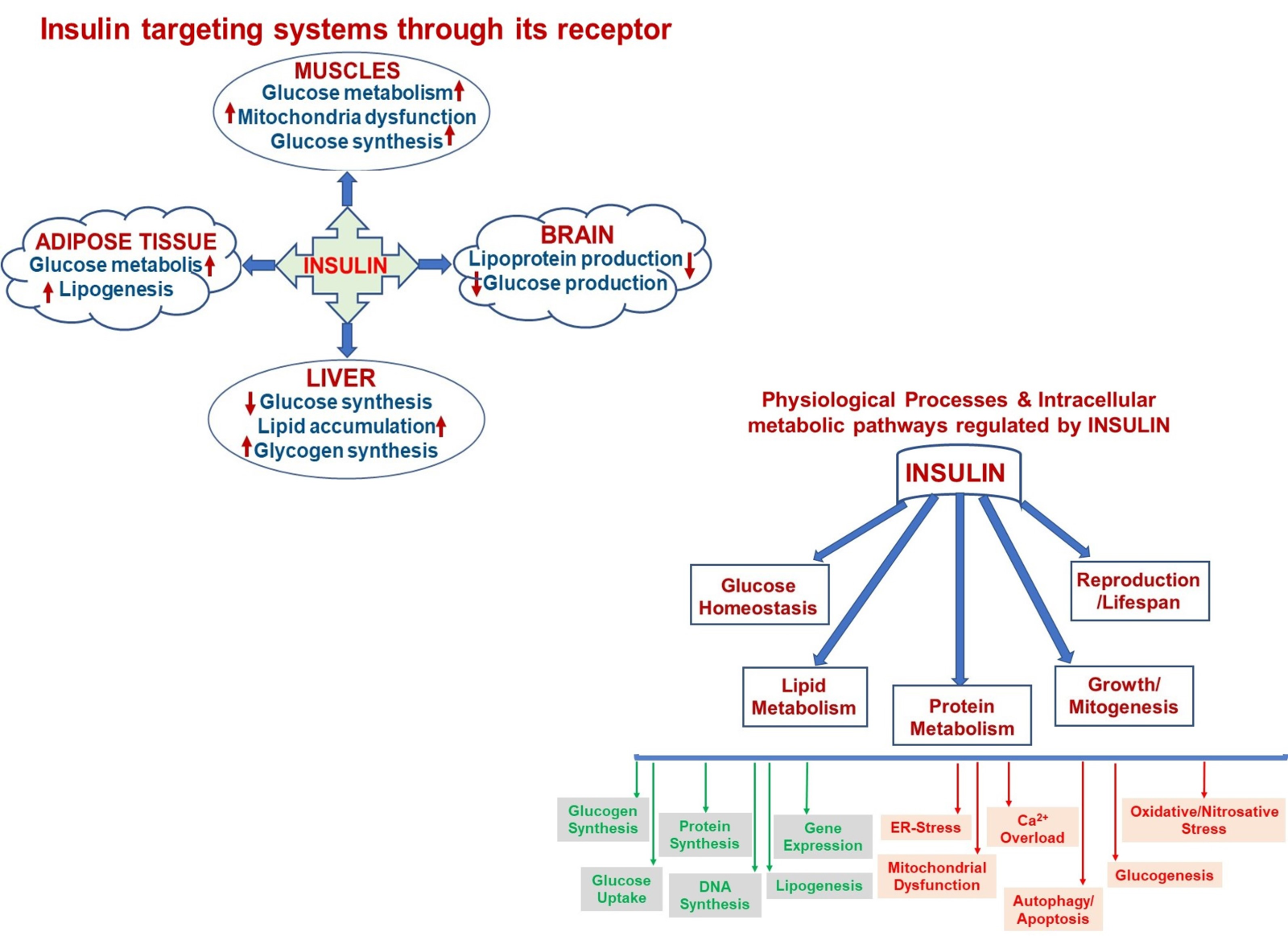
In a new window | Download PPT
Figure 2: A schematic representation of insulin targeting systems in the body through its receptor (left) and multiple physiological processes in the organism regulated by insulin via affecting various intracellular metabolic pathways either as by increasing or decreasing manner.
Although previous experimental studies examined the contribution of the regulatory roles of insulin/IGF-signaling in aging heart dysfunction (Inuzuka et al., 2009; Kenyon, 2005; Wessells et al., 2004), the undelying mechanism is not well understood yet. Wessells et al. (2004) studied the regulatory role of insulin on heart function in aging fruit flies and demonstrated that insulin-IGF signaling influences age-dependent organ physiology and senescence directly and autonomously, in addition to its systemic effect on lifespan. In further review articles, the role of insulin/IGF signaling in preserving cardiac performance in the aged mammalian heart was discussed (Boudina, 2013; Lee and Kim, 2018). In addition, it is well known that the absence of insulin signaling in the heart induces changes in voltage-dependent K+-channel expression/function and prolongation in AP duration of isolated ventricular cells, parallel to long-QT in mammalian heart (Lopez-Izquierdo et al., 2014). Although these results support the above notation that the leading cause of insulin signaling disturbance in the heart is repolarization abnormalities, including long-QT, in animal models of diabetes as well as aged-mammals (Bertrand et al., 2008; Durak et al., 2018; Olgar et al., 2020a; Olgar et al., 2020b), it is not exactly known whether or not there is a direct insulin effect on cardiac performance in aged mammalians. Some experimental studies have demonstrated the efficacy of insulin in preventing long-QT and the associated arrhythmias in diabetic animals through reduction of the rapid delayed rectifier K+-current (Zhang et al., 2006). These results support the hypothesis that lack of insulin signaling can produce abnormal repolarization in cardiomyocytes and the development of the arrhythmogenic potential, further leading to an increase in the incidence of sudden cardiac death. Supporting this hypothesis, recently, Olgar et al. (2021b), for the first time, demonstrated that insulin provided important benefits on long QT in insulin-resistant aged rats by accelerating the ventricular action potential-repolarization by reversing inhibited KCNQ1/KCNE channel-current (IKs) through the beta adrenergic receptor subtype 3 (β3-ARs) signaling. Indeed, the protective role of insulin therapy as an adjunct to reperfusion after ischemia in the heart has been demonstrated with insulin directly promoting myocardial cell survival independent of any effects on metabolic modulation (Sack and Yellon, 2003). Later studies showed that insulin is a key component of the GIK-cocktail (glucose-insulin-potassium) that modulates the PI3K-Akt-eNOS-dependent signal mechanism (Alburquerque-Béjar et al., 2015; Boucher et al., 2014; Lopez-Izquierdo et al., 2014; Yu et al., 2011). Taking into consideration the role of cAMP and PKA activation on IKs (Kanda et al., 2011), one can hypothesize their contribution to this current. However, previous studies demonstrated that both cyclic adenosine monophosphate (cAMP) and protein kinase A were found to be depressed in insulin-resistant hearts, in a manner parallel to activation of β3-AR (Dincer et al., 2001; Okatan et al., 2015).
To show whether insulin therapy has cardioprotective action in the aging heart, aged rats (24-mo-old) were treated with insulin for two weeks, and then their heart function was evaluated using micro-computer tomography images (Fig. 3A). The summarized data are presented in Fig. 3B. As can be seen in this graph, most of the altered heart parameters were reversed by insulin therapy. Both in vivo and in vitro studies strongly indicate the cardioprotective action of insulin in the aged heart is by reversing the inhibited IKs, which acts as an atypical activator normalizing the long QT in insulin-resistant aged rats by accelerating the ventricular AP repolarization (Olgar et al., 2021b). There are also early findings supporting recent data in which authors demonstrated the contribution of changes in KCNQ1/KCNE channels to AP duration and repolarization in mammalian heart preparations (Abbott, 2020; Dixit, Dabney-Smith, & Lorigan, 2020; Huang et al., 2018; Marx et al., 2002). An earlier supporting study found the regulation of KCNQ1 is performed by non-KjnjCNE protein subunits, such as 𝛽AR-signaling hub (Marx et al., 2002). Correspondingly the recent data, demonstrating a direct beneficial effect of insulin on the depressed IKs, as determined in either insulin resistant aged or metabolic syndrome rats could answer the question of whether insulin can have a protective effect on the aging heart.
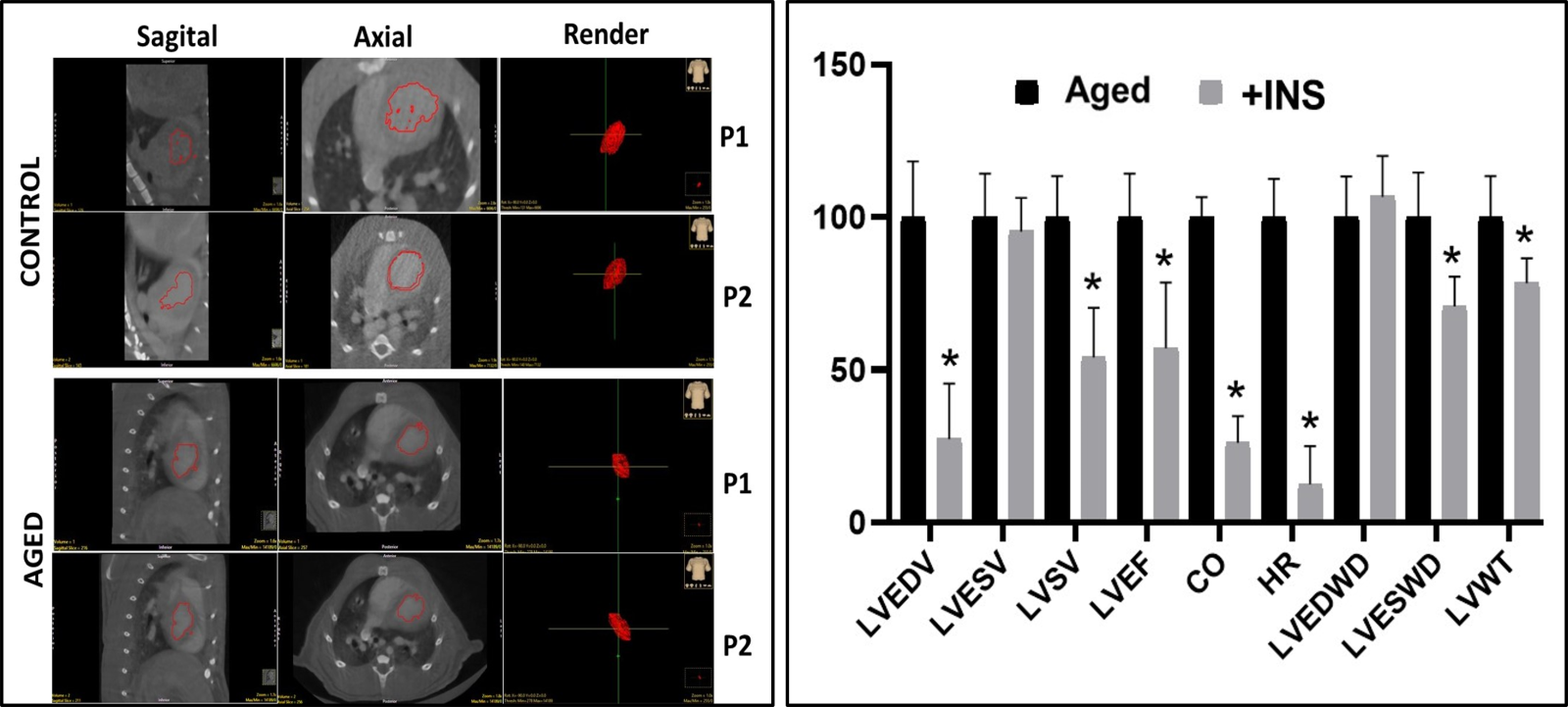
In a new window | Download PPT
Figure 3: Representative ex-vivo micro-CT scans (echocardiographic) of heart from aged rat in comparison to the adult rat heart and the % change in the insulin-treated aged rats. (A) The aged is the heart of a 24-mo-old male rat, while the control is the heart of an adult rat (8-mo- old male). Representative high-definition 3D segmentation images generated by ex-vivo micro-CT where volumes 1 and 2 represent diastole and systole phases in the hearts (P01: diastole and P02: systole). (B) The aged rats are treated with insulin (+INS; 2 IU/kg/day, for two weeks) and then their ex-vivo micro-CT images were quantified and presented as % changes with respect to the untreated aged rats. LVESV: Left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), left ventricular stroke volume (LVSV), left ventricular ejection fraction (LVEF), cardiac output (CO), heart rate (HR), left ventricular end-diastolic wall diameter (LVEDWD), left ventricular end- systolic wall diameter (LVESWD), and left ventricular left ventricular wall thickness (LVWT). The values are presented as mean (±SEM) for every parameter for hearts and *p<0.05 vs. untreated Aged group.
Beneficial effects of insulin on mitochondria function in aged rat ventricular cardiomyocytes
Generally, it has been well documented the impact of high mitochondrial reactive oxygen species (ROS) production in the pathogenesis of insulin resistance. Early experimental studies have shown an inhibitory role of tumor necrosis factor-alpha (TNF-alpha) on insulin induced increases on serine phosphorylation of IR substrate (IRS) proteins, thereby decreasing tyrosine phosphorylation of IRS proteins (Kanety, 1995). With cell level examinations, it has been reported that TNF-α can increase mitochondrial ROS production in tumor cells, while hyperglycemia increased mitochondrial ROS production in endothelial cells (Corda et al., 2001; Kukidome et al., 2006; Nishikawa et al., 2000). Supporting data also demonstrated that this TNF-α-induced ROS over production was suppressed by overexpression of manganese superoxide dismutase (MnSOD) (Imoto et al., 2006). In these regards, further experimental studies performed in insulin-resistant aged rat heart have demonstrated that there are significantly high levels of mitochondrial superoxide and Ca2+, which are major activators of mitochondrial permeability transition pore opening, and increases in the mRNA level of Bnip3, which has important roles in cell death, autophagy, mitophagy, myocardial stiffness, and SR and mitochondrial Ca2+-homeostasis (Chaanine et al., 2013). More importantly, incubation of ventricular cardiomyocytes isolated from aged rat heart with a mitochondria-targeting antioxidant significantly attenuated most of these alterations (Olgar et al., 2020a).
Recently it has been demonstrated that insulin treatment can provide significant cardioprotection against insufficient-heart function in elderly mammals with myocardial insulin resistance by reversing long QT, particularly affecting dysfunctional KCNQ1/KCNE1-channels. Similar benefits were also obtained with a protein kinase G (PKG) inhibitor under both in vitro and in vivo applications. Furthermore, inhibited IKs in β3-ARs-stimulated cells could be reversed with a PKG inhibitor, indicating the correlation between activated PKG and β3-ARs activation. These data further imply a strong relationship between the inhibited IKs in aged-rat cardiomyocytes and activated β3-ARs, via the associated interaction between KCNQ1 and KCNE1. Moreover, the effect of insulin therapy on the co-localization of KCNQ1 and KCNE1 in the isolated left ventricular cardiomyocytes from insulin-resistant aged rats has also been examined. As can be seen in Fig. 4A, their co-localization is not significantly different from those of untreated aged rats, whereas co-immunoprecipitation analysis demonstrated the benefits of insulin treatment in the aged rats on the co-localization of KCNQ1 and KCNE1 (Olgar et al., 2021b).
There are multiple factors that contribute to the development of dysfunction in the aging heart, Among them, excessive mitochondrial ROS production associated with mitochondrial functional and structural changes are causally linked to the pathophysiology of aging in the heart (Bou-Teen et al., 2021; Olgar et al., 2020a; Olgar et al., 2020b). Furthermore, the role of mitochondrial dysfunction in insulin resistance is already documented by several good studies in mammal hearts (Kim et al., 2008). Indeed, it has been also well documented why altered mitochondrial metabolism is the underlying basis for the increased sensitivity in the aged heart to stress (Lesnefsky et al., 2016). To validate these findings, the levels of ROS and mitochondrial membrane potential (MMP), were determined with confocal microscopy in an aging-modeled rat ventricular cell line (H9c2 cells) by incubating these cells with D-galactose (50 mg/mL for 48-h incubation) with and without insulin. As can be seen in Fig. 4B, the increased levels of both ROS and MMP could be reversed by treating the aged cardiomyoctes with insulin treatment (24-h with 100 nM).
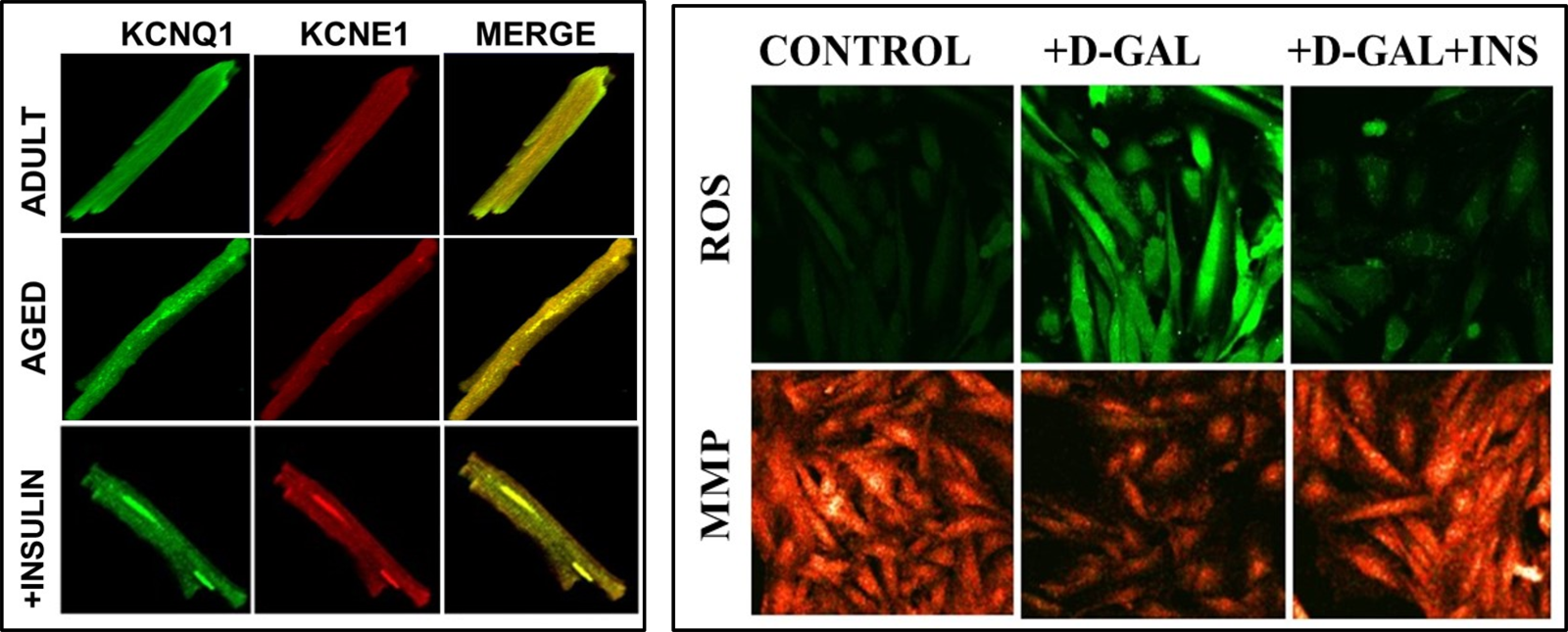
In a new window | Download PPT
Figure 4: Representative confocal images to demonstrate the colocalization of KCNQ1 and KCNE1, and reactive oxygen species (ROS) and mitochondrial membrane potential (MMP) values in ventricular cells. (A) Colocalization of KCNQ1 and KCNE1 in adult and aged rat cardiomyocytes with and without insulin treatment (2 IU/kg/day, for two weeks) at room temperature, as described previously (Olgar et al., 2021). All cells were fixed and permeabilized and then incubated with specific primary antibodies and were followed by secondary antibodies. The cells were mounted in a medium containing DAPI (blue to stain nuclei). (B) The representative confocal images of the cells to demonstrate the levels of ROS imaging with DCFDA (10-μM for 60-min loading) in insulin-treated cells (100 nM for 24-h) with respect to untreated cells.To monitor the ROS level, the DCFDA loaded cells were calibrated with H2O2 (100-μM). MMP imaging with JC-1 (5-μM for 30-min loading) inD-Gal induced aging-mimicked H9c2 cell line with insulin-treated cells (100 nM for 24-h) with respect to untreated cells (Olgar et al., 2019). The probes were excited at 488 nm, and the red fluorescence image was detected at both 535 and 585 nm. To calibrate the changes in MMP,cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, 5-μM) was used.
In cardiac myocytes, mitochondria are particularly abundant and are specialized in subcellular populations. The present and previously published results strongly emphasize the role of defective mitochondria in the persistent pathological stimuli in the aged heart, which lead to enhanced oxidant production, oxidative injury, and the activation of oxidant signaling for cell death. Understanding the mechansim(s) underlying the direct protective action of insulin on mitochondria could provide additional information that may lead to new therapies that target aging defects in the heart. These mechanisms may provide new ways to attenuate cardiac disease preemptively in the elderly by treating age-related defects, in contrast to treatment disease-induced dysfunction.
In addition, previous literature provide important and strong evidence emphasizing the cross-relation between insulin and mitochondrial function. In this regards, the process of energy metabolism in muscles, similar to other tissues, is linked to mitochondrial function and also insulin action (Schellet al., 2021). The heart is a high energy demand organ, and it mostly depends on functional insulin/insulin-like growth factor (IGF)-1 signaling in the cardiovascular system. The required high energy is mainly provided by mitochondria in the form of ATP, consequently, there is cross-interplay between mitochondrial function and insulin/IGF-1 signaling to perform the required metabolic signaling for proper continuous heart function. Besides the action of insulin at the organ/tissue level, it has important actions at the cellular level. Insulin activates its receptor on the cell membrane, which is related to IGF-1 receptor (Kleinridders, 2016), and induces two important downstream signaling pathways such as the mitogen-activated protein kinase-extracellular signal-regulated kinase (ERK) signaling sub-pathway and phosphoinositide 3-kinase/protein kinase B (AKT)-signaling sub-pathways. Both of these two pathways play a role in cell proliferation, gene transcription, and modulation of metabolic effects including protein and lipid synthesis (Boucher et al., 2014). These downstream signaling targets of insulin play vital roles in mitochondria associated cell metabolism, leading to proper organ function (Cai et al., 2018; García-Cáceres et al., 2016; Logan et al., 2018).
Consequently, the findings of experimental studies clearly reveal that IR/IGF-1R interaction with mitochondrial function in organs, including the heart is crucial for cellular energy homeostasis and proper energy metabolism. Thus, experiments examining insulin or insulin-like agents as a treatment modality in any aging animal model, will be a novel approach to targeting cardiovascular disease in the elderly (Olgar et al., 2020a; Olgar et al., 2021b).
Concluding remarks
Cardiac aging is a progressive and intrinsic decline in heart function characterized by a long QT together with reduced heart rate and depressed contractile activity. Recently, a relationship between impairment in insulin signaling and aging leading to heart failure and sudden cardiac arrest has been noted. With the continuously growing elderly population worldwide, there is a great need for interventions in cardiac aging. This article provides recent findings detailing some important targets for insulin in cardiac aging such as IKs and mitochondria. An understanding of these parameters with further studies will present additional knowledge regarding the molecular mechanisms underlying these types of changes including channelopathies and organellopathies, and more novel advances in the development of interventions to delay or reverse cardiac aging.
Acknowledgments
I would like to thank my team members in Ankara University Faculty of Medicine, Department of Biophysics for their contributions. This study was supported by The Scientific and Technological Research Council of Turkey (SBAG-119S661) to Belma Turan.
Conflict of interests
The author declares that there is no conflict of interest.
References
Belma Turan
Department of Biophysics, Lokman Hekim University Faculty of Medicine, Ankara, Turkey.
Corresponding author:
Prof. Belma Turan, PhD
Email: belma.turan@medicine.ankara.edu.tr & belma.turan@lokmanhekim.edu.tr

In a new window | Download PPT
Figure 1: Whole-cell TTX-sensitive voltage-dependent Na+-channel currents were recorded in freshly isolated ventricular cardiomyocytes from male Wistar rats with an age of 24-mo-old (Aged group) and age of 8-mo-old (Adult group) as described, previously (Bilginoglu et al., 2013). Freshly cardiomyocyte isolation was performed by using the enzymatic method, as described, previously (Olgar et al., 2021). For comparison, all recorded currents were divided by the cell membrane capacitance to present them as current density (in pA/pF). The frequency of the maximum amplitude variation with membrane potentials is presented as histograms for adult (left) and aged (right) groups, respectively. The frequency of the maximum amplitude of the aged group did shift to the right (positive potentials) starting from -40 mV in comparison to the adult group, implying the existence of late Na+-currents.

In a new window | Download PPT
Figure 2: A schematic representation of insulin targeting systems in the body through its receptor (left) and multiple physiological processes in the organism regulated by insulin via affecting various intracellular metabolic pathways either as by increasing or decreasing manner.

In a new window | Download PPT
Figure 3: Representative ex-vivo micro-CT scans (echocardiographic) of heart from aged rat in comparison to the adult rat heart and the % change in the insulin-treated aged rats. (A) The aged is the heart of a 24-mo-old male rat, while the control is the heart of an adult rat (8-mo- old male). Representative high-definition 3D segmentation images generated by ex-vivo micro-CT where volumes 1 and 2 represent diastole and systole phases in the hearts (P01: diastole and P02: systole). (B) The aged rats are treated with insulin (+INS; 2 IU/kg/day, for two weeks) and then their ex-vivo micro-CT images were quantified and presented as % changes with respect to the untreated aged rats. LVESV: Left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), left ventricular stroke volume (LVSV), left ventricular ejection fraction (LVEF), cardiac output (CO), heart rate (HR), left ventricular end-diastolic wall diameter (LVEDWD), left ventricular end- systolic wall diameter (LVESWD), and left ventricular left ventricular wall thickness (LVWT). The values are presented as mean (±SEM) for every parameter for hearts and *p<0.05 vs. untreated Aged group.

In a new window | Download PPT
Figure 4: Representative confocal images to demonstrate the colocalization of KCNQ1 and KCNE1, and reactive oxygen species (ROS) and mitochondrial membrane potential (MMP) values in ventricular cells. (A) Colocalization of KCNQ1 and KCNE1 in adult and aged rat cardiomyocytes with and without insulin treatment (2 IU/kg/day, for two weeks) at room temperature, as described previously (Olgar et al., 2021). All cells were fixed and permeabilized and then incubated with specific primary antibodies and were followed by secondary antibodies. The cells were mounted in a medium containing DAPI (blue to stain nuclei). (B) The representative confocal images of the cells to demonstrate the levels of ROS imaging with DCFDA (10-μM for 60-min loading) in insulin-treated cells (100 nM for 24-h) with respect to untreated cells.To monitor the ROS level, the DCFDA loaded cells were calibrated with H2O2 (100-μM). MMP imaging with JC-1 (5-μM for 30-min loading) inD-Gal induced aging-mimicked H9c2 cell line with insulin-treated cells (100 nM for 24-h) with respect to untreated cells (Olgar et al., 2019). The probes were excited at 488 nm, and the red fluorescence image was detected at both 535 and 585 nm. To calibrate the changes in MMP,cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, 5-μM) was used.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6256 | 5 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA