Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
NCX as a key player in the neuroprotection exerted by ischemic preconditioning and postconditioning
Time:2019-03-07
Number:1615
Pignataro Giuseppe1
Author Affiliations


- 1Division Pharmacology, Department of Neurosciences, Reproductive and Odontostomatological Sciences, School of Medicine, “Federico II” University of Naples, Italy
5th International Symposium on Conditioning Medicine. November, 2018.
Abstract
Although the mechanisms through which pre- and post-conditioning exert their effects are not yet fully understood, since the early ‘90s, numerous research groups, including the one headed by Elizabeth Murphy, have hypothesized and demonstrated that the protection induced by ischemic preconditioning in the heart occurred through the control of sodium and calcium ion homeostasis. Therefore, ischemic preconditioning seems to strengthen the whole cellular machinery required for the maintenance of ionic homeostasis (Steenbergen et al., 1993).
This working hypothesis, initially developed through studies conducted on the heart, seems to be valid also for all the other organs, including the brain.
In this context, we demonstrated that Na+/Ca2+exchangers (NCXs), a family of ionic membrane transporters that contribute to the maintenance of intracellular ionic homeostasis and contribute to the progression of the ischemic lesion, play a key role in propagating these neuroprotective phenomena.
Interestingly, we have previously demonstrated that NCX1 and NCX3, two of the three brain isoforms of the plasmamembrane Na+/Ca2+exchanger, are novel additional targets for the survival action of the (PI3-K)/Akt pathway (Formisano et al., 2008). In fact, AKT functions as a major downstream target of phosphatidylinositol 3-kinase (PI3-K), and after phosphorylation, it phosphorylates some substrates on the serine or threonine residues, including glycogen synthase kinase-3, Caenorhabditis elegans DAF-16 transcription factor, Bad, phosphodiesterase 3B, and the tuberous sclerosis complex-2 tumor suppressor gene product tuberin (Chan,2004).
Over the years we accumulated evidence showing that the two NCX isoforms, NCX1 and NCX3, might take part as effectors in the neuroprotection evoked by preconditioning and postconditioning.
In particular, our data support the importance of p-AKT in mediating preconditioning neuroprotection and suggest that NCX1 and NCX3 are indeed two additional signals downstream of p-AKT that are involved in the neuroprotective process of ischemic preconditioning. On the other hand, p-AKT should not be considered the only transducer able to activate NCX1 and NCX3. In fact, numerous other cellular factors are most likely released even in earlier stages, as for instance right after preconditioning induction, and can therefore control the levels of NCX expression. In this respect, we have recently demonstrated that after ischemic preconditioning HIF-1ais strongly augmented. This increase, in turn, is accompanied by an increase in NCX1 expression, which contributes to brain preconditioning neuroprotection (Valsecchi etal., 2011). These results demonstrate that ncx1 gene is a novel HIF-1 target and that HIF-1 exerts its pro- survival role also through NCX1 up-regulation during brain preconditioning. Therefore, HIF-1, at least in part, exerts its neuroprotective effect by inducing an overepression of NCX1 that is mediated by the interaction between HIF-1 and NCX1 promoter (Valsecchi et al., 2011). In 2015, an epigenetic regulation of sodium/calcium exchanger isoform 1 (NCX1), respectively, by two functional protein complexes was reported: REST/Sp3/HDAC1/HDAC2 and HIF-1/Sp1/p300. In particular, whereas the former downregulates NCX1 expression during brain ischemia, the latter upregulates it during preconditioning. Notably, the development of drugs that epigenetically regulate NCX1 by preventing its downregulation in stroke might be a new pharmacological avenue to ameliorate neuronal damage during brain ischemia (Formisano et al., 2015; Formisano et al.,2013).
As concern the role played by NCX during brain postconditioning, we demonstrated that among the three NCX isoforms expressed in the CNS, NCX3 represents an additional new molecular effector involved in the neuroprotection exerted by ischemic postconditioning. In particular, in our experimental model of ischemic postconditioning, obtained by subjecting adult male rats to 10 minutes of subliminal tMCAO applied 10 minutes after 100 minutes of tMCAO, we provided solid evidence showing that p-AKT is the mediator of this action since (1) p-AKT expression after postconditioning increased and timely mirrors that of NCX3; (2) NCX3 downregulation, induced by siRNA, reverts the neuroprotection induced by ischemic postconditioning; and (3) the selective p-AKT inhibition prevents NCX3 up-regulation thus reverting the postconditioning-induced neuroprotection (Pignataro et al., 2011).
Notably, this transporter seems to play a relevant role also in other neurological disorders such as Amyotrophic Lateral Sclerosis (Anzilotti et al., 2018). Indeed, the use of the toxin L-BMAA at subliminal concentrations, is able to indce an amelioration of clinical conditions of ALS mice by enhancing NCX3 expression in spinal cord(Anzilotti et al., 2018).
Data on NCX highlighted the role of NCX1 and NCX3 in mediating neuroprotection elicited by preconditioning and postconditioning thus suggesting that an effective stroke therapy could be designed by inducing an overexpression of these two NCX isoforms or by increasing their activity.
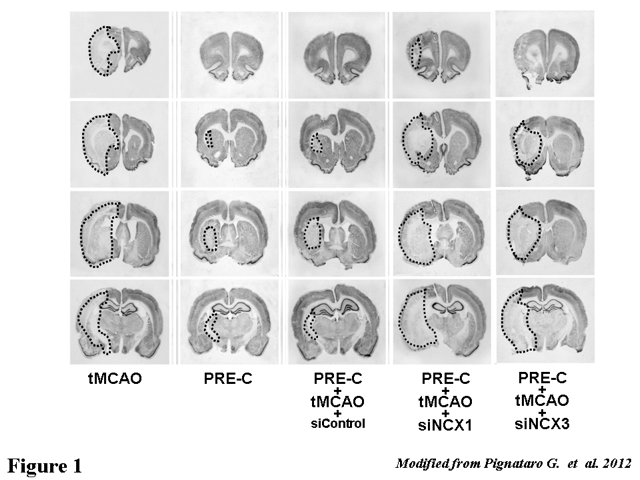
Figure 1. Effect of NCX1 and NCX3 silencing on Ischemic Preconditioning-induced neuroprotection. Representative coronal brain slices, stained with NeuN, of rats subjected to preconditioning (PreC) followed by harmful ischemia (tMCAO) and treated with siRNA control (siControl), siRNA against NCX1(siNCX1) or siRNA against NCX3 (siNCX3). The ischemic area is circled.
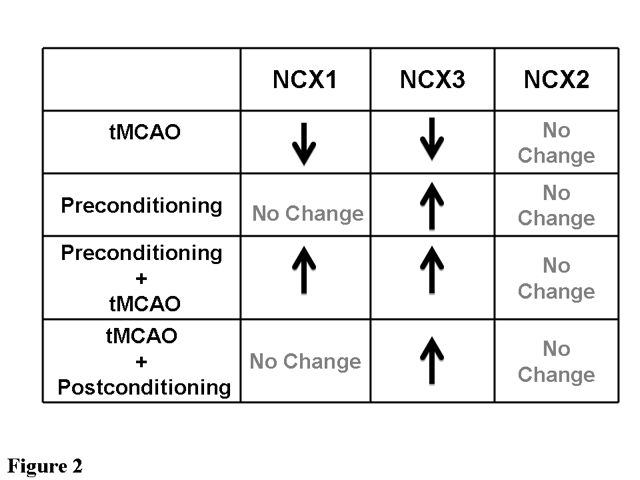
Figure 2. Modifications in NCX1, NCX2 and NCX3 expression after tMCAO, preconditioning; preconditioning plus tMCAO and tMCAO plus postconditioning.
References
- Anzilotti S, Brancaccio P, Simeone G, Valsecchi V, Vinciguerra A, Secondo A, et al. (2018). Preconditioning, induced by sub-toxic dose of the neurotoxin L-BMAA, delays ALS progression in mice and prevents Na(+)/Ca(2+) exchanger 3 downregulation. Cell Death Dis 9(2):206.
- Chan PH (2004). Future targets and cascades for neuroprotective strategies. Stroke35(11 Suppl 1): 2748-2750.
- Formisano L, Guida N, Valsecchi V, Cantile M, Cuomo O, Vinciguerra A, et al. (2015). Sp3/REST/HDAC1/HDAC2 Complex Represses and Sp1/HIF-1/p300 Complex Activates ncx1 Gene Transcription, in Brain Ischemia and in Ischemic Brain Preconditioning, by Epigenetic Mechanism. J Neurosci 35(19):7332-7348.
- Formisano L, Guida N, Valsecchi V, Pignataro G, Vinciguerra A, Pannaccione A, et al. (2013). NCX1 is a new rest target gene: role in cerebral ischemia. Neurobiol Dis 50:76-85.
- Formisano L, Saggese M, Secondo A, Sirabella R, Vito P, Valsecchi V, et al. (2008). The two isoforms ofthe Na+/Ca2+ exchanger, NCX1 and NCX3, constitute novel additional targets for the prosurvival action of Akt/protein kinase B pathway. Mol Pharmacol 73(3):727-737.
- Pignataro G, Esposito E, Cuomo O, Sirabella R, Boscia F, Guida N, et al. (2011). The NCX3 isoform of the Na(+)/Ca(2+) exchanger contributes to neuroprotection elicited by ischemic postconditioning. J Cereb Blood FlowMetab.
- Steenbergen C, Perlman ME, London RE, Murphy E (1993). Mechanism of preconditioning. Ionic alterations. Circ Res72(1):112-125.
- Valsecchi V, Pignataro G, Del Prete A, Sirabella R, Matrone C, Boscia F, et al. (2011). NCX1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke 42(3):754-763.
Abstract
Although the mechanisms through which pre- and post-conditioning exert their effects are not yet fully understood, since the early ‘90s, numerous research groups, including the one headed by Elizabeth Murphy, have hypothesized and demonstrated that the protection induced by ischemic preconditioning in the heart occurred through the control of sodium and calcium ion homeostasis. Therefore, ischemic preconditioning seems to strengthen the whole cellular machinery required for the maintenance of ionic homeostasis (Steenbergen et al., 1993).
This working hypothesis, initially developed through studies conducted on the heart, seems to be valid also for all the other organs, including the brain.
In this context, we demonstrated that Na+/Ca2+exchangers (NCXs), a family of ionic membrane transporters that contribute to the maintenance of intracellular ionic homeostasis and contribute to the progression of the ischemic lesion, play a key role in propagating these neuroprotective phenomena.
Interestingly, we have previously demonstrated that NCX1 and NCX3, two of the three brain isoforms of the plasmamembrane Na+/Ca2+exchanger, are novel additional targets for the survival action of the (PI3-K)/Akt pathway (Formisano et al., 2008). In fact, AKT functions as a major downstream target of phosphatidylinositol 3-kinase (PI3-K), and after phosphorylation, it phosphorylates some substrates on the serine or threonine residues, including glycogen synthase kinase-3, Caenorhabditis elegans DAF-16 transcription factor, Bad, phosphodiesterase 3B, and the tuberous sclerosis complex-2 tumor suppressor gene product tuberin (Chan,2004).
Over the years we accumulated evidence showing that the two NCX isoforms, NCX1 and NCX3, might take part as effectors in the neuroprotection evoked by preconditioning and postconditioning.
In particular, our data support the importance of p-AKT in mediating preconditioning neuroprotection and suggest that NCX1 and NCX3 are indeed two additional signals downstream of p-AKT that are involved in the neuroprotective process of ischemic preconditioning. On the other hand, p-AKT should not be considered the only transducer able to activate NCX1 and NCX3. In fact, numerous other cellular factors are most likely released even in earlier stages, as for instance right after preconditioning induction, and can therefore control the levels of NCX expression. In this respect, we have recently demonstrated that after ischemic preconditioning HIF-1ais strongly augmented. This increase, in turn, is accompanied by an increase in NCX1 expression, which contributes to brain preconditioning neuroprotection (Valsecchi etal., 2011). These results demonstrate that ncx1 gene is a novel HIF-1 target and that HIF-1 exerts its pro- survival role also through NCX1 up-regulation during brain preconditioning. Therefore, HIF-1, at least in part, exerts its neuroprotective effect by inducing an overepression of NCX1 that is mediated by the interaction between HIF-1 and NCX1 promoter (Valsecchi et al., 2011). In 2015, an epigenetic regulation of sodium/calcium exchanger isoform 1 (NCX1), respectively, by two functional protein complexes was reported: REST/Sp3/HDAC1/HDAC2 and HIF-1/Sp1/p300. In particular, whereas the former downregulates NCX1 expression during brain ischemia, the latter upregulates it during preconditioning. Notably, the development of drugs that epigenetically regulate NCX1 by preventing its downregulation in stroke might be a new pharmacological avenue to ameliorate neuronal damage during brain ischemia (Formisano et al., 2015; Formisano et al.,2013).
As concern the role played by NCX during brain postconditioning, we demonstrated that among the three NCX isoforms expressed in the CNS, NCX3 represents an additional new molecular effector involved in the neuroprotection exerted by ischemic postconditioning. In particular, in our experimental model of ischemic postconditioning, obtained by subjecting adult male rats to 10 minutes of subliminal tMCAO applied 10 minutes after 100 minutes of tMCAO, we provided solid evidence showing that p-AKT is the mediator of this action since (1) p-AKT expression after postconditioning increased and timely mirrors that of NCX3; (2) NCX3 downregulation, induced by siRNA, reverts the neuroprotection induced by ischemic postconditioning; and (3) the selective p-AKT inhibition prevents NCX3 up-regulation thus reverting the postconditioning-induced neuroprotection (Pignataro et al., 2011).
Notably, this transporter seems to play a relevant role also in other neurological disorders such as Amyotrophic Lateral Sclerosis (Anzilotti et al., 2018). Indeed, the use of the toxin L-BMAA at subliminal concentrations, is able to indce an amelioration of clinical conditions of ALS mice by enhancing NCX3 expression in spinal cord(Anzilotti et al., 2018).
Data on NCX highlighted the role of NCX1 and NCX3 in mediating neuroprotection elicited by preconditioning and postconditioning thus suggesting that an effective stroke therapy could be designed by inducing an overexpression of these two NCX isoforms or by increasing their activity.

Figure 1. Effect of NCX1 and NCX3 silencing on Ischemic Preconditioning-induced neuroprotection. Representative coronal brain slices, stained with NeuN, of rats subjected to preconditioning (PreC) followed by harmful ischemia (tMCAO) and treated with siRNA control (siControl), siRNA against NCX1(siNCX1) or siRNA against NCX3 (siNCX3). The ischemic area is circled.

Figure 2. Modifications in NCX1, NCX2 and NCX3 expression after tMCAO, preconditioning; preconditioning plus tMCAO and tMCAO plus postconditioning.
References
- Anzilotti S, Brancaccio P, Simeone G, Valsecchi V, Vinciguerra A, Secondo A, et al. (2018). Preconditioning, induced by sub-toxic dose of the neurotoxin L-BMAA, delays ALS progression in mice and prevents Na(+)/Ca(2+) exchanger 3 downregulation. Cell Death Dis 9(2):206.
- Chan PH (2004). Future targets and cascades for neuroprotective strategies. Stroke35(11 Suppl 1): 2748-2750.
- Formisano L, Guida N, Valsecchi V, Cantile M, Cuomo O, Vinciguerra A, et al. (2015). Sp3/REST/HDAC1/HDAC2 Complex Represses and Sp1/HIF-1/p300 Complex Activates ncx1 Gene Transcription, in Brain Ischemia and in Ischemic Brain Preconditioning, by Epigenetic Mechanism. J Neurosci 35(19):7332-7348.
- Formisano L, Guida N, Valsecchi V, Pignataro G, Vinciguerra A, Pannaccione A, et al. (2013). NCX1 is a new rest target gene: role in cerebral ischemia. Neurobiol Dis 50:76-85.
- Formisano L, Saggese M, Secondo A, Sirabella R, Vito P, Valsecchi V, et al. (2008). The two isoforms ofthe Na+/Ca2+ exchanger, NCX1 and NCX3, constitute novel additional targets for the prosurvival action of Akt/protein kinase B pathway. Mol Pharmacol 73(3):727-737.
- Pignataro G, Esposito E, Cuomo O, Sirabella R, Boscia F, Guida N, et al. (2011). The NCX3 isoform of the Na(+)/Ca(2+) exchanger contributes to neuroprotection elicited by ischemic postconditioning. J Cereb Blood FlowMetab.
- Steenbergen C, Perlman ME, London RE, Murphy E (1993). Mechanism of preconditioning. Ionic alterations. Circ Res72(1):112-125.
- Valsecchi V, Pignataro G, Del Prete A, Sirabella R, Matrone C, Boscia F, et al. (2011). NCX1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke 42(3):754-763.
References
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 1615 | 0 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA