International bi-monthly journal of cell signaling, tissue protection, and translational research.
[Special Issue] Aging, obesity, and male: Co-morbidities and treatments of COVID-19
Chase Kingsbury1, Jeffrey Farooq1, Nadia Sadanandan2, Blaise Cozene3, You Jeong Park1, Zhen-Jie Wang1, Justin Cho1, Bella Gonazalez-Portillo4, Madeline Saft5, Mia C. Borlongan6, Jea-Young Lee1
Author Affiliations


- 1Department of Neurosurgery and Brain Repair, University of South Florida Morsani. College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, USA.
- 2Georgetown University, 3700 O St NW, Washington, DC 20057, USA.
- 3Tulane University: 6823 St. Charles Ave, New Orleans, LA 70118, USA.
- 4Northwestern University, 633 Clark St, Evanston, IL 60208, USA.
- 5University of Michigan, 500 S State St, Ann Arbor, MI 48109, USA.
- 6University of California Berkeley, 101 Sprout Hall, Berkeley, CA 94720, USA.
Abstract
There seems to be no end to the ongoing battle against the COVID-19 pandemic. According to the World Health Organization, there are over a million confirmed deaths across the globe and at least 35 million confirmed cases of COVID-19 today; CDC reports a total of 7.5 million COVID-19 cases with approximately 200,000 deaths in the United States alone. Additionally, comorbidities, such as age, weight, and gender, affect morbidity and mortality rates, with the aged, obese, and male populations being more susceptible to the virus. Currently, there are hundreds of developing COVID-19 vaccines with only six currently in phase III clinical trials. Alternative remedies, such as remdesivir, dexamethasone, and REGN-COV2, have been applied as COVID-19 treatments for the time being. This review will discuss the current state of the ongoing pandemic, the effects of co-morbidities, as well as the status of promising drugs such as remdesivir, dexamethasone, and REGN-COV2.
Keywords: coronavirus, aging, obesity, male, drugs, vaccines
Abstract
There seems to be no end to the ongoing battle against the COVID-19 pandemic. According to the World Health Organization, there are over a million confirmed deaths across the globe and at least 35 million confirmed cases of COVID-19 today; CDC reports a total of 7.5 million COVID-19 cases with approximately 200,000 deaths in the United States alone. Additionally, comorbidities, such as age, weight, and gender, affect morbidity and mortality rates, with the aged, obese, and male populations being more susceptible to the virus. Currently, there are hundreds of developing COVID-19 vaccines with only six currently in phase III clinical trials. Alternative remedies, such as remdesivir, dexamethasone, and REGN-COV2, have been applied as COVID-19 treatments for the time being. This review will discuss the current state of the ongoing pandemic, the effects of co-morbidities, as well as the status of promising drugs such as remdesivir, dexamethasone, and REGN-COV2.
Keywords: coronavirus, aging, obesity, male, drugs, vaccines
Introduction
Coronavirus disease 2019 (COVID-19), closely related to betacoronaviruses Severe Acute Respiratory Syndrome Coronavirus (SARS-Cov) and Middle East Respiratory Syndrome-related Coronavirus (MERS-Cov), has taken the world by surprise (Park et al., 2020). As of June, there has been reported over 8 million confirmed cases and around 450,000 deaths globally (Park et al., 2020). In the United States alone, there have been about 2.1 million confirmed cases and around 120,000 deaths (Park et al., 2020). Since then, these numbers of infections and fatalities have increased significantly. COVID-19 is caused by the novel coronavirus, SARS-Cov-2, and shares similar pulmonary deficits as SARS-Cov and MERS-Cov (Sariol and Perlman, 2020). However, in the 2003 SARS and 2012 MERS epidemics, very limited human-to-human transmission was observed, ~8,100 and 2,500 confirmed cases respectively (Park et al., 2020; Sariol and Perlman, 2020). The S1 subunit of the spike protein present on the surface of the novel coronavirus has a high binding affinity with the angiotensin-converting enzyme 2 (ACE2) receptor on human cells present in the lung, heart, and other vital organs. This may be a key factor present in COVID-19 that explains its virulence relative to previous coronaviruses. Given the infectious nature of COVID-19 relative to past coronaviruses, significant research has targeted the predisposition of comorbidities and the development of effective vaccines and other treatments such as dexamethesone, remdesivir, and REGN-COV-2 (Hotez et al., 2020).
Co-morbidities presenting in COVID-19
Certain comorbidities may increase vulnerability to COVID-19. Pre-existing conditions, such as chronic respiratory illness, immune/inflammatory disorders, diabetes, and hypertension represent the most implicated COVID-19 comorbidities (Du et al., 2020; Korakas et al., 2020; Richardson et al., 2020). Chronic obstructive pulmonary disease (COPD) was identified as a significant anticipatory factor for admittance to the intensive care unit (ICU) following SARS-CoV-2 infection (Jain et al., 2020). COVID-19 in conjunction with COPD culminated in exacerbated clinical outcome (Jain et al., 2020). Similarly, autoimmune disorders stand as routine presenting comorbidity disease in selected patients admitted to ICU, with 87% of these patients exhibiting pneumonia-like complications (Montero et al., 2020). Notably, the male biological sex amplified disease severity in COVID-19 patients with pre-existing respiratory illness and the use of glucocorticoids correlated with ICU admission (Montero et al., 2020). Other comorbidities present in COVID-19 patients include aging, obesity, and sex. Mortality rate with COVID-19 infection was highest in men older than 50, who also suffered from pre-existing conditions (Du et al., 2020). Elderly individuals were at high-risk for developing COVID-19 due to their impaired immune system (Perrotta et al., 2020). Older individuals displayed immunosenescence, the deterioration of the immune system as a result of age, which hinders the immune system’s ability to clear the SARS-CoV-2 virus from the body (Perrotta et al., 2020). COVID-19 patients exhibiting a higher body mass index (BMI) more frequently associated with critical cases and deaths (Yadav et al., 2020). More than 80% of the patients who died due to COVID-19 displayed a high BMI (Yadav et al., 2020). Obesity led to a chronic inflammatory state since obese individuals have a greater concentration of pro-inflammatory cytokines. The pro-inflammatory cytokines impair the responses of B and T cells, which results in higher susceptibility to the virus as well as delayed resolution (Yadav et al., 2020). Moreover, sex-disparity was apparent in COVID-19 cases. The likelihood of death due to COVID-19 was higher for men in every age category (Punjani et al., 2020). The rates of case positivity, hospitalization, and death were higher for men when compared to women for every age category (Punjani et al., 2020). Such gender-related disparity suggests a role of androgen or sex hormones; however, further research is warranted to explore the cause of this sex disparity (Punjani et al., 2020)). Moreover, the heightened inflammatory state associated with immune-deficiency disorders, aging, obesity, and the male sex may further exacerbate the cytokine storm induced by SARS-CoV-2 infection, indicating why these comorbidities may render individuals more vulnerable to COVID-19.
Clinical trials of vaccines
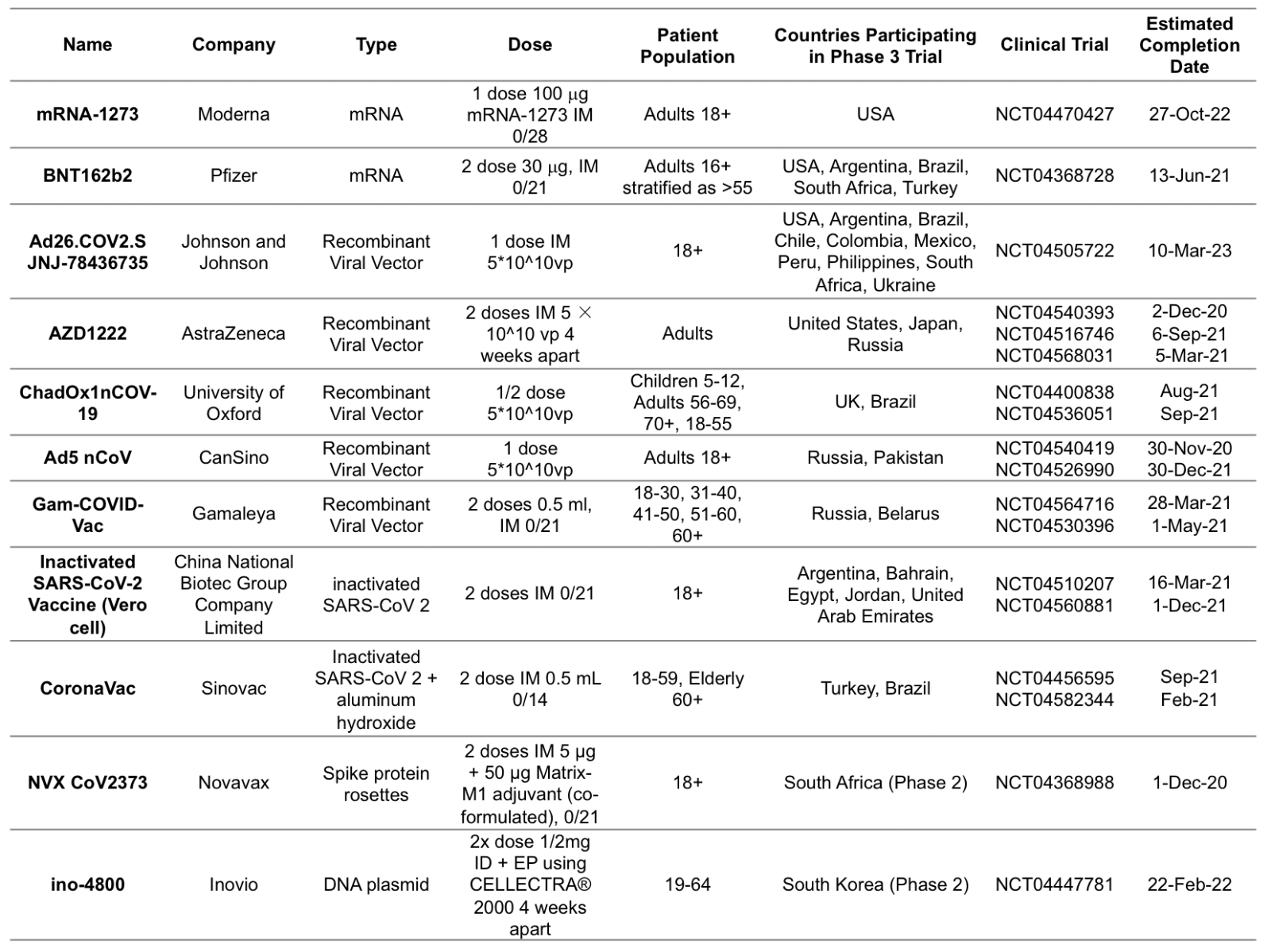
Since the start of the pandemic, 44 vaccines have initiated clinical trials in humans globally, with at least 92 preclinical vaccines under active investigation (Poland et al., 2020). In the USA, there are four vaccines in phase III trials (Table 1). The mRNA-1273 vaccine manufactured by Moderna contains a lipid nanoparticle encapsulated messenger RNA that encodes the S-2P antigen, consisting of the SARS-CoV-2 glycoprotein with a transmembrane anchor and an intact S1–S2 cleavage site. This vaccine induced anti-SARS-CoV-2 immune responses in all participants measured by enzyme-linked immunosorbent assay anti–S-2P antibody geometric mean titer (GMT) in phase I/II clinical trials. Systemic adverse events occurred in the highest dose (250-μg) group, with three participants reporting one or more severe adverse events, however no trial-limiting safety concerns were identified, and phase III trials were initiated with an anticipated primary completion in October 2022 (Jackson et al., 2020). Pfizer is developing two lipid nanoparticle−formulated, nucleoside−modified RNA vaccine candidates BNT162b1 and BNT162b2. BNT162b1 encodes a secreted trimerized SARS-CoV-2 receptor-binding domain, and BNT162b2 encodes a prefusion stabilized membrane-anchored SARS−CoV−2 full-length spike. In phase I clinical trials the two vaccine candidates elicited similar dose-dependent SARS-CoV-2-neutralizing GMTs, comparable to or higher than the GMT of a panel of SARS-CoV-2 convalescent sera in both younger and older adults; however, BNT162b2 was less systemically reactogenic, particularly in older adults, leading to BNT162b2’s selection for combined phase II/III trials (Mulligan et al., 2020). Astra Zeneca and Pfizer have developed recombinant vaccine candidates for the Sars-Cov 2 spike protein based on the ChadOx viral vector (Folegatti et al., 2020; Graham et al., 2020). There are currently no existing nucleic acid based vaccines approved for use outside of medical research, but they are a promising technology for investigation, as they can be produced with only the nucleic acid sequence of viral antigens, thus simplifying the design and manufacturing stages of vaccine production (Chauhan et al., 2020). Viral vector vaccines contain recombinant viruses, usually adenoviruses, that are unable to replicate but produce recognizable antigens for the immune system. However, there are concerns of immunogenicity to the viral vector itself, which would inhibit antigen production and re-immunization (Enjuanes et al., 2016). Inactivated/weakened virus vaccines require significantly more expenditure to cultivate live virus particles in suitable media. In addition, live-attenuated viruses are efficacious in eliciting an immune response, though there is a risk of reversion to a virulent state (Chen, 2020). Other countries including China, Russia, and the United Kingdom are also conducting phase III clinical trials of vaccines. CanSino’s Ad5-nCoV was the first vaccine candidate to be approved for emergency use outside of phase III trials. Astra Zeneca suspended trials for AZD1222 when a member of the trial cohort developed transverse myelitis (Mallapaty et al., 2020). Sinovac’s Coronavac vaccine has been prophylactically administered to workers traveling abroad, though this is outside of monitored clinical trials, and the effects of this unprecedented widespread use are currently unknown (Chung YH et al., 2020; Van Norman, 2020). It seems likely that unanticipated adverse effects will occur given the rapid pace of vaccine development and complex disease pathology of COVID-19. The preliminary results of candidate-vaccine clinical trials are promising for combating the pandemic, but there are still some safety and efficacy concerns that must be addressed in these final phases of testing.
Remdesivir, Dexamethasone, and REGN-COV2
Two prominent drugs currently approved for COVID-19 therapy are dexamethasone and remdesivir, and a third experimental drug, REGN-COV2, is pending approval contingent upon the results of ongoing clinical trials. Dexamethasone is an immunosuppressant corticosteroid that modulates lymphocyte proliferation, cytokine release, and systemic inflammation by increasing the production of anti-inflammatory interleukin 10 (IL-10), inhibiting the release of the pro-macrophage factor interferon gamma, and downregulating synthesis of phospholipase A2 and cyclooxygenase-2 (Faccioli et al., 1990; Verhoef et al., 1999; Yu et al., 2020). It is approved for the treatment of a broad spectrum of medical conditions including rheumatoid arthritis, bacterial meningitis, asthma, post-surgical sequelae, and cancer (de Gans and van de Beek, 2002; Kroot et al., 2006; Keeney et al., 2014; Wang et al., 2016). Dexamethasone is particularly effective at suppressing autoimmunity in rheumatoid arthritis and mitigating the inflammatory and broncho-constrictive pathology associated with lung tissue damage in asthma. It was originally produced in 1958 by MERCK as an oral tablet in doses ranging from 0.25 mg to 6 mg, but it is now manufactured off-patent by more than a dozen pharmaceutical companies globally in oral, intramuscular, and intravenous forms for less than $0.33 per dose. The current interest in dexamethasone for COVID-19 therapy derives from its widespread availability, success in ameliorating lung damage, and an excellent safety profile (Li et al., 2018; Xu et al., 2020). The RECOVERY Trial was designed in response to this interest and is an ongoing randomized, controlled, multi-arm study that is currently evaluating the efficacy of dexamethasone as a therapeutic for COVID-19 (The RECOVERY Collaborative Group, 2020). Preliminary findings in 2,104 patients suggest that 6 mg of oral dexamethasone administered for up to 10 days increases the survival rate in ventilator- and oxygen-dependent patients compared to patients receiving standard care. The US Food and Drug Administration approved the use of dexamethasone under these conditions in these patients based on these results. The CoDEX randomized clinical trial similarly determined that a five-day regimen of 20 mg intravenous dexamethasone followed by five days of 10 mg dexamethasone reduces the number of ventilator-dependent days in critically ill patients (Tomazini et al., 2020). Numerous smaller studies corroborate these findings while also indicating that corticosteroids do not have any impact or may even worsen outcomes in stable COVID-19 patients (Fadel et al., 2020; Fernandez-Cruz et al., 2020; Nelson et al., 2020; Sterne et al., 2020; Wu et al., 2020). Therefore, dexamethasone appears to be most beneficial in treating acute, critically-ill patients with co-morbidities associated with ventilator-dependence such as the elderly and those with underlying asthma. Remdesivir, or GS-5734, is a nucleoside analog synthesized by Gilead Sciences Inc. that has shown antiviral activity against several RNA virus families like the Filoviridae (Ebola and Marburg virus), Paramyxoviridae (respiratory syncytial, Nipah, and Hendra virus), and Coronaviridae (SARS-CoV and MERS-CoV) in preclinical studies (Warren et al., 2016; Lo et al., 2017; Sheahan et al., 2017; Sheahan et al., 2020). As a prodrug, remdesivir requires activation by host cell triphosphate and competitively binds to viral RNA-dependent RNA polymerase. Given its efficacy against the Ebola virus in non-human primates (Warren et al., 2016), intravenous remdesivir was explored in a randomized, multi-intervention trial following an Ebola outbreak in the Democratic Republic of Congo (Mulangu et al., 2019). Despite its success in preclinical studies, patients receiving remdesivir fared the worst of the treatment groups, however the trial did confirm its safety. Interest in remdesivir for COVID-19 stems from an in vitro study that highlighted remdesivir’s potential against SARS-CoV-2 (Wang et al., 2020), and the US Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the treatment of COVID-19 with remdesivir via the compassionate use program (Pardo et al., 2020). A cohort study of a 10-day treatment of remdesivir (200 mg dose on day 1 followed by 100 mg dose daily in the subsequent days) prescribed in a compassionate-use basis in 53 patients hospitalized with COVID-19 with oxygen saturation of 94% or less showed clinical improvement in 36 patients (68%) as defined by oxygen-support status (Grein et al., 2020). Mortality was at 13% and associated with age 70 or greater, higher serum creatinine, and invasive ventilation. Larger clinical trials of remdesivir for COVID-19 has been promising but highlights remdesivir alone may not be sufficient for all patients. A double-blind, randomized, placebo-controlled trial of intravenous remdesivir (200 mg dose on day 1 followed by 100 mg dose daily for up to 9 additional days) in 1063 patients hospitalized with COVID-19 showed that the drug was superior to placebo in reducing the time to recovery (Beigel et al., 2020). However, a double-blind, randomized, placebo-controlled multicenter trial looking at 237 patients with severe COVID-19 in Hubei, China showed that there was no difference in time to clinical improvement between patients receiving remdesivir or placebo. Of note, patients with symptom duration of 10 days or less treated with remdesivir have a faster time to clinical improvement, though the results were not statistically significant (Wang et al., 2020). A randomized, open-label multicenter trial looking at 533 patients with moderate COVID-19 being treated with a 10-day course of remdesivir (200 mg dose on day 1 followed by 100 mg dose daily in the subsequent days), 5-day course, or standard care showed no statistically significant difference in clinical status between the 10-day course treatment group and standard care group. These results suggest remdesivir may be useful in combination with other drugs, and future strategies include use with immune modulators (Beigel et al., 2020). A third promising drug candidate for COVID-19 therapy is REGN-COV2, a cocktail of two fully human monoclonal antibodies manufactured by Regeneron Pharmaceuticals intended to confer resistance to the virus. These neutralizing antibodies form high-affinity bonds with the receptor binding domain of SARS-CoV-2’s spike protein to impede viral host-cell entry (Hansen et al., 2020). The combination of two spike protein-specific antibodies prevents mutational escape, the phenomenon where small genetic variations in the viral RNA allows SARS-CoV-2 to evade binding when only a single antibody is administered (Baum et al., 2020). The results of a small, randomized clinical trial that administered REGN-COV2 as a one-time infusion of 8 mg or 2.4 mg demonstrated significantly reduced viral load and symptomatic improvement, particularly in patients with higher baseline viral levels. Furthermore, the treatment augmented the recovery of patients who already developed endogenous antibodies to SARS-CoV-2, implying it may also be beneficial to recovering patients. Importantly, REGN-COV2 was only investigated in the outpatient setting, and there are now five clinical trials, including the comprehensive RECOVERY trial, currently enrolling to further explore its effects.
Conclusion
Comorbidities significantly exacerbate mortality and morbidity rates of COVID-19 patients. Overwhelming evidence indicates that age, weight, and gender, specifically the elderly, obese, and male, are among the most predisposed to infection. With millions of positive cases across the globe, vaccines still remain in clinical trials. Before approval for public administration, vaccines must demonstrate efficacy in preventing infection and no adverse reactions due to the large population of vulnerable individuals presenting with comorbidities and old age (Kaur and Gupta, 2020). These factors contribute to the delayed release of vaccines to the public, and with only 6 vaccines in phase III clinical trials, there is an estimated public release in 2021. It is predicted that worldwide administration may take longer once a vaccine is approved. Despite the long road to a vaccine, the use of dexamethasone as a treatment for critical condition COVID-19 patients has displayed promising results (Wahab et al., 2020). Similarly, remdesivir has demonstrated efficacy in vitro although clinical efficacy has not been established (Akhtar et al., 2020). Lastly, REGN-COV-2, a cocktail of two antibodies (REGN10987+REGN10933), has demonstrated efficacy in vivo by decreasing the viral load in the upper and lower airways in rhesus macaques, and reducing weight loss and implications of pneumonia in hamsters (Baum et al., 2020). The scientific community continues to provide evidence-based clinical treatments of COVID-19 (Akhtar et al., 2020).
Conflicts of interest statement:
The authors declare no conflicts of interest.
Acknowledgement:
The authors thank their colleagues at University of South Florida Morsani College of Medicine, Georgetown University, Tulane University, Northwestern University, University of Michigan, and University of California Berkeley for the critical discussion of this manuscript.
References
Chase Kingsbury1*
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani. College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, USA.
Jeffrey Farooq1*
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani. College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, USA.
Nadia Sadanandan2*
2Georgetown University, 3700 O St NW, Washington, DC 20057, USA.
Blaise Cozene3*
3Tulane University: 6823 St. Charles Ave, New Orleans, LA 70118, USA.
You Jeong Park1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani. College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, USA.
Zhen-Jie Wang1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani. College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, USA.
Justin Cho1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani. College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, USA.
Bella Gonazalez-Portillo4
4Northwestern University, 633 Clark St, Evanston, IL 60208, USA.
Madeline Saft5
5University of Michigan, 500 S State St, Ann Arbor, MI 48109, USA.
Mia C. Borlongan6
6University of California Berkeley, 101 Sprout Hall, Berkeley, CA 94720, USA.
Jea-Young Lee1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani. College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, USA.
*These authors contribute equally to this article.
Corresponding author:
Mia C. Borlongan
Email: mborlong@berkeley.edu)
or
Jea-Young Lee
Email: jeayoung@usf.edu
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5492 | 13 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA